The following image is an example of resonance structures. Determine the most stable resonance structure. The answer could be more than one of the options.
A B C
What is A and B?
R and S is a system of differentiating the orientation of stereocenters. Determine the configuration of the starred stereocenter of the following molecule.
What is S?
Predict the following compound in each pair that will undergo the Sn2 reaction faster.
A B
What is compound B?
The following reaction is E1. What are the missing reactants?
What is H2SO4 and heat?
The presence of this functional group is indicated by a distinct stretch at 3300 AND a stretch at 2200 cm-1.
What is an alkyne?
The following molecule has this IUPAC name.
What is pent-1-en-3-ol ?
The relationship between the following molecules (with respect to isomerism) can be described as what?
What is diastereomers?
The following substance, when reacting with an alkene, performs what kind of reaction? Also, what kind of product does it result in with respect to this reaction?
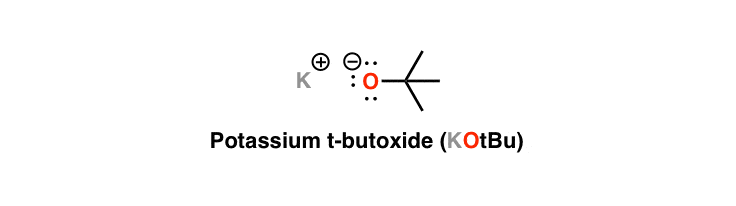
What is E2, Hoffman product?
For which of the following types of reactions is rearrangement possible?
A. Acid Catalyzed Hydration
B. Sn2
C. Sn1
D. E2
E. Hydroboration-oxidation
Determine how many signals would appear in HNMR AND C-NMR for this molecule.
What is 4 HNMR signals and 4 CNMR signals?
The Newman projection is a method of drawing a molecule in order to visualize it three dimensionally. The following Newman projection is of butane, the four carbon alkane. Give the name for the positioning of the groups coming off of the middle carbons.

What is "gauche?"
The following molecules are what relationship with respect to stereochemistry?

What is enantiomers?
Transformation of a secondary or tertiary ROH to RX is which of the following types of reactions? (One correct answer).
E1, Sn1, Sn2, or E2?
What is Sn1?
Give the unknown reactants needed for the following reaction to occur.
What is CH2X3, NaOH, and H2O?
Determine which functional groups are present in this molecule based on the following IR spectroscopy reading.
What is an aldehyde? (And CH SP3)
Chair conformations are drawn in order to show the position of cyclohexane substituents. The substituents that experience lower energy because they are gauche to other substituents and no longer experience 1,3 diaxial interactions are at WHAT POSITION?
What is the equatorial position?
Name the following molecule using the Cahn-Ingold-Prelog convention.
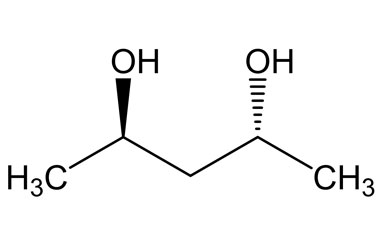
What is (2R, 4R)-Pentane-2,4-diol?
The conjugate acid of a compound is always a weaker ___________ than the base. (The answer in the blank is defined as a molecule/substance that has a tendency to DONATE electrons at electron poor sites).
What is nucleophile?
Name two reactions that both add TWO -OH groups. (The fashion in which they add the OH groups is the difference between the reactions). You must give ALL the reactants for both for any points.
What is Anti Dihydroxylation (reactants are mCPBA/ peroxic acid and H+ and H2O), and Syn Dihydroxylation (Reactants are EITHER KMnO4 cold dilute and -OH, H2O... or OsO4 and H2O2).
An unknown compound has a distinct sharp stretch around 2200 wavenumbers on the IR spectroscopy graph. What two groups could this indicate and how would one be able to determine which of these two groups the molecule possesses?
What is alkyne or nitrile. The compound possesses the alkyne if there is also evidence of C-H stretching with sp hybridization around the 3300 wavenumber, then the unknown molecule possesses an alkyne.
Free radical halogenation is a means of replacing a hydrogen atom on a carbon molecule with a halogen. It is initiated by heat/light. This reaction results in a halogenated organic compound. Answer the following questions in order regarding this reaction.
This step of free radical halogenation is the only step that starts with a radical and ends with a radical.
This step starts with no radicals and ends with a radical.
What is the steps of propagation and initiation?
There are asymmetric carbons within this molecule. Identify how many.
Then, identify whether the molecule is chiral or achiral.
What is one asymmetric carbon (middle carbon) and molecule is chiral?
The image below shows carbocations. Determine which is the most stable.
What is E?
The following reaction depicts the synthesis of an alkyne. Please give the two missing reactants.
What is KOH (fused) and heat?
Determine the functional groups present given the following molecule's IR and Mass Spectrometry.
What is Nitrile, Aromatic, and CH Sp3?