This element is the primary component of organic molecules and is tetravalent.
What is Carbon-14?
The formula for ethanal.
What is C2H4O?
The number of carbons in hexane.
What is 6?
Benzene and an R group.
What are the components of an arene?
The type of reaction occurring when an alkene reacts with a halogen.
What is an addition reaction?
The type of reaction between an alkane and a halogen.
The name of an 6 carbon chain with a double bond between carbon 2 and 3.
What is hex-2-ene?
The functional group and ending added to a molecule with an OH- group.
What is an alcohol, -ol?
Hydrocarbons with only single bonds, also known as saturated hydrocarbons.
What are alkanes?
The product of the reaction between propene and H2 in the presence of a Ni-catalyst.
What is propane?
A reaction in which two molecules react to form a product with the loss of a small molecule.
What is a condensation reaction?
The name of a 5-carbon hydrocarbon with a methyl group on carbon #2.
What is a 2-methylpentane?
This functional group adds the suffix -anoate to the end of molecule's name.
The product of gentle oxidation of a secondary alcohol.
What is a ketone?
The reaction conditions used to collect an aldehyde from oxidation of a primary alcohol.
What is distillation?
The products of a complete oxidation reaction.
What is H2O and CO2?
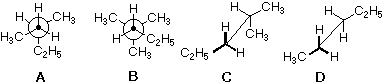
The structure of 2-methylpentane.
What is C?
What is the resulting prefix of saturated single bond carbon rings?
What is cyclo-?
The two reagents of an ester.
What are Alcohol and Carboxylic Acid?
The reaction conditions used to make a carboxylic acid form a primary alcohol.
What is reflux?
It is often used as a reactive in chemical assays as a reagent to distinguish between alkanes and alkenes using a halogenation mechanism.
What is Bromine Water? (Br2 dissolved in H2O)
Pentane, 2-methylbutane and 2,2-dimethylpropane are the 3 structural isomers of this formula.
What is C5H12?
These two elements with a double bond results in the prefix, nitroso-.
What is nitrogen and oxygen?
The difference between a thial and a thioal.
What is a double bond for thial, single bond for thiol?
The name of the reaction mechanism when a bond breaks by splitting the shared pair of electrons equally between the two products.
What is homolytic fission?