Name the following compound:
CH3CH2CH2CH2CH3 (C5H12)
Pentane
Draw propan-2-ol

Name this compound:
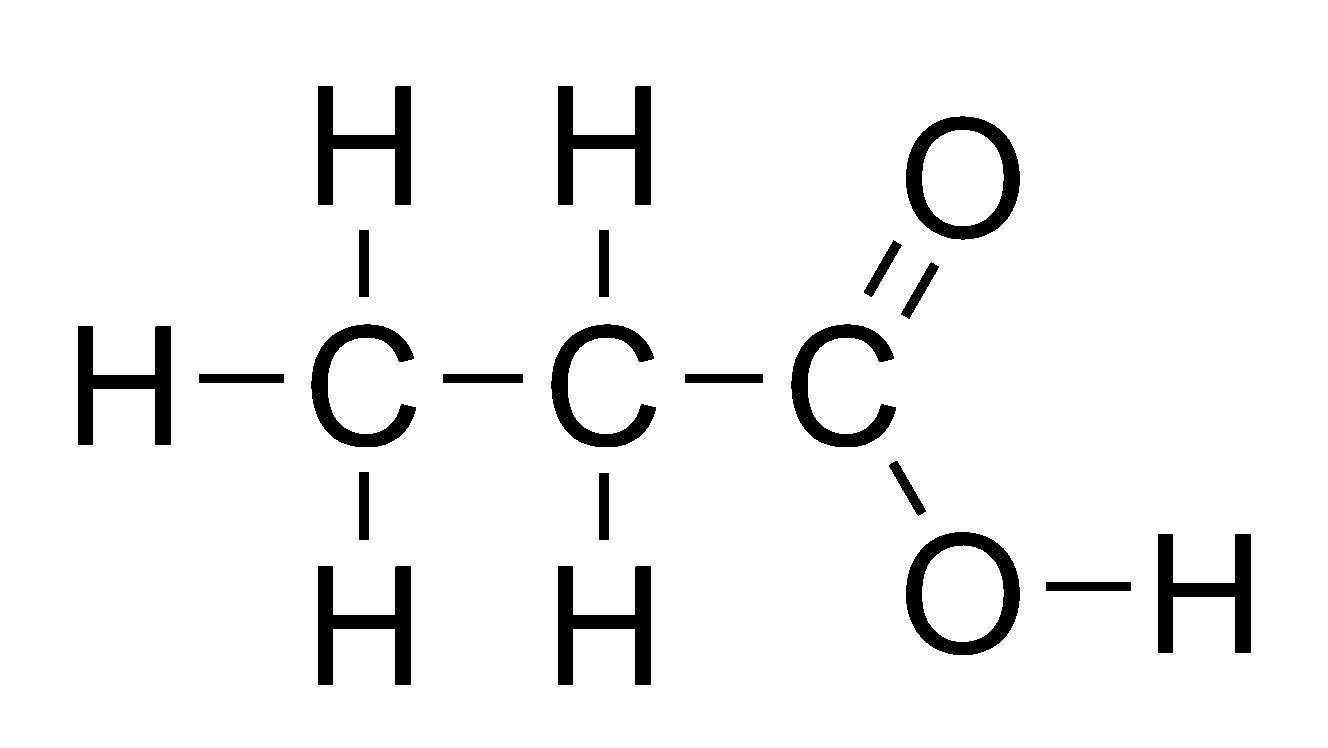
Propanoic Acid
Which end of a soap molecule is hydrophobic - the carbon chain tail or the charged ion head?

Carbon chain tail
Polymers can be classified as either addition or ________ polymers
Condensation
This type of bond exists where electrons are being shared between the carbon atoms and the carbon-hydrogen atoms in an alkane.
Covalent bonds
What is the name of the alcohol functional group (-OH)
Hydroxl
What two reactants are needed to make pentyl butanoate?
1- Pentanol and Butanoic Acid
Name a chemical that a triglyceride fat could react with to produce a soap?
Sodium Hydroxide (or other Group 1 Hydroxide)
What is the feature of monomers which allows them to undergo addition polymerisation?
They have a double bond in the carbon chain
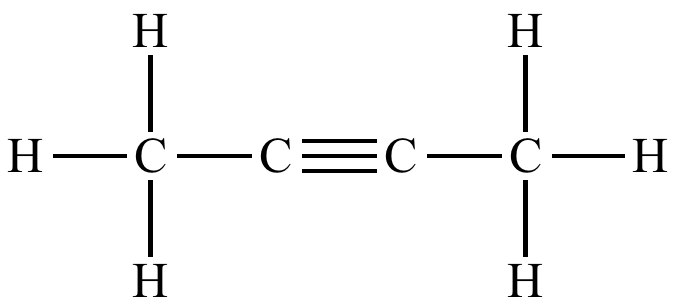
Draw 2-butyne
Which type of alcohol will not undergo an oxidation reaction?
Tertiary Alcohol
What is the name of the reaction setup for producing an ester where you use an upright condenser?
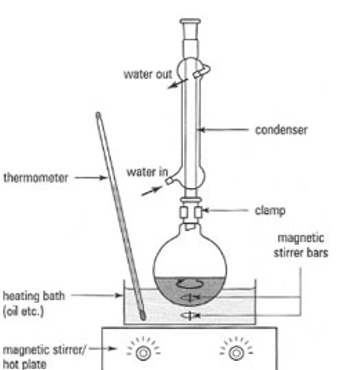
Reflux
What is the name given to the soap structures formed when they embed their tails into grease, leaving the charged ionic heads to be attracted to polar water?
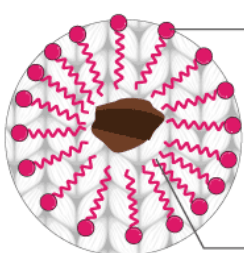
Micelle
The following molecule is apidic acid. Is it a monomer for addition or condensation polymerisation?
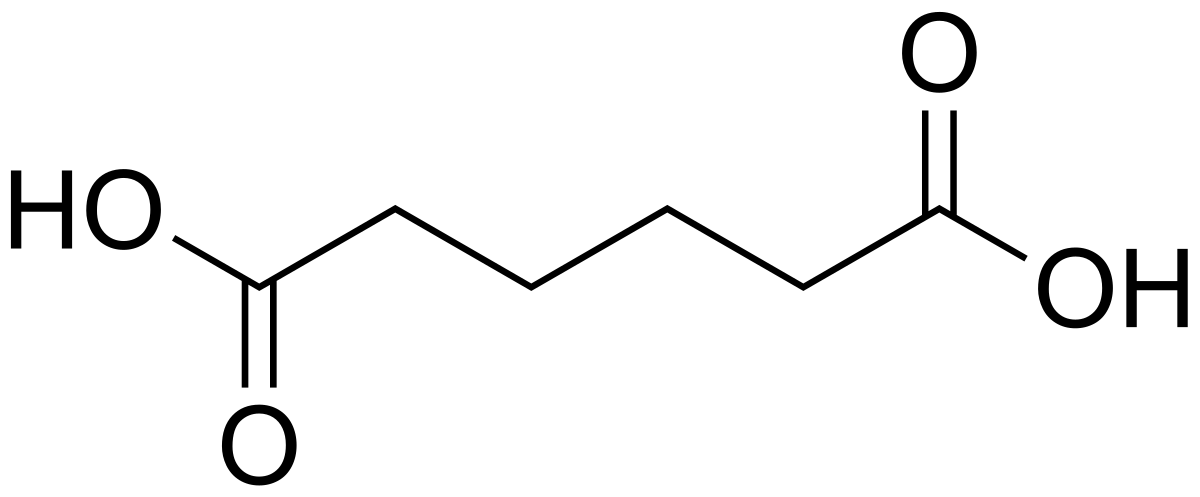
Condensation (Functional groups on both ends)
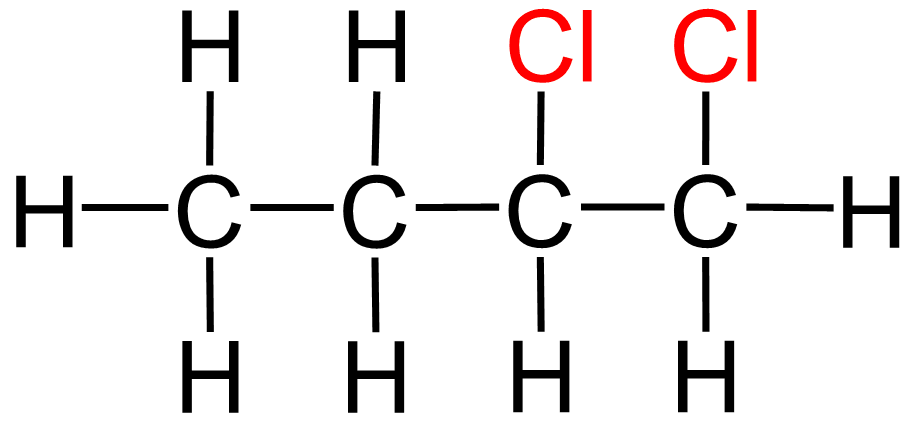
Name this compound:
1,2 - dichloroethane
What needs to be added to ethene to produce ethanol, and what catalyst is needed?
Water, with a phosphoric acid catalyst.
Name the following ester:
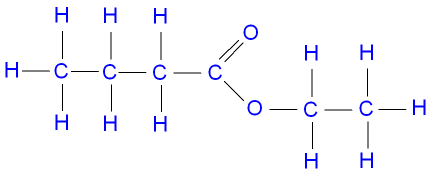
Ethyl butanoate
What is the structure of a cationic detergent?
Carbon chain tail and a positive ionic head.
What is the IUPAC name of this monomer, and the common name of the polymer it forms?

Chloroethene, Polyvinylchloride (PVC)
Explain why the boiling points of the alkane homologous series increase as the number of carbons increase.
Boiling point is related to the Intermolecular Forces. As the carbon chain gets longer, there is more dispersion force between the molecules so the boiling point increases.
Give the reaction steps needed to get from ethane to ethanol.
Ethane undergoes a substitution reaction with Cl2 to produce chloroethane. This then undergoes a substitution reaction with NaOH to produce ethanol.
Describe, using names of the functional groups, what is occurring to make the ester bond between an acid and an alcohol.
The hydrogen comes away from the organic acid in the carboxyl functional group. The hydroxl group comes away from the alcohol. This creates a bond site on both the acid and alcohol, and they join forming the ester bond.
Describe the difference between a natural soap and an anionic detergent
Natural soap has a carboxyl ionic head. An anionic detergent will have another negative ion as the head.
What structural features of high density polyethylene allow it to form a strong and slightly flexible plastic?
High dispersion forces between long, straight polyethylene chains.