Do organic molecules tend to have high or low boiling points?
Usually low.
Draw the correct line angle (skeletal) formula for 1-ethyl-4-methylcyclohexane.
What would this type of branch/substituent be called?
CH3-CH2-CH2-CH2-CH2---
Pentyl.
How does a carboxylic acid differ from an ester?
A carboxylic acid has a OH located next to a C=O group and is found on a terminal end of a carbon chain, versus an ester has a O located next to the C=O and is in the middle of a carbon chain.
What is the name of this functional group?
R - OH
Alcohol.
How many bonds can a carbon atom form?
4, based on the fact that it has 4 valence electrons.
What would be the correct line angle (skeletal) formula be for the compound 2,4-dimethylhexane?
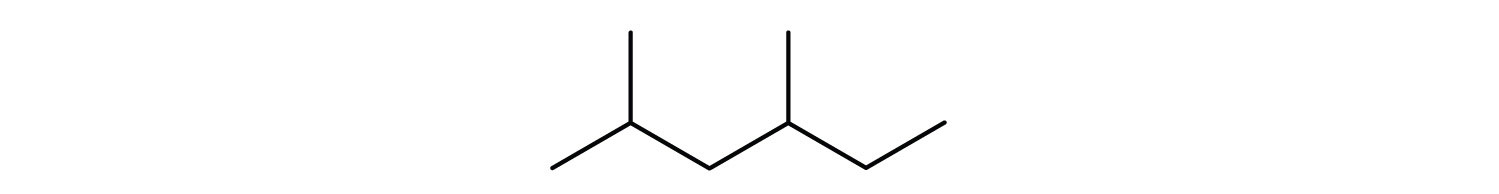
Name the following compound:

Methylcyclononane.
Which functional groups contain a carbonyl group?
Aldehyde, ketone, carboxylic acid, ester, amide.
What atom joins two alkyls (carbon groups) to form an ether?
Oxygen
What is the general molecular formula for an alkane?
CnH2n+2.
What is the correct CONDENSED structural formula for 5,6-diethyl-2-methylnonane


1-chloro-2,2-dimethylpropane
How does an amine differ from an amide?
An amine is a nitrogen single bonded to a carbon chain, and amide is a nitrogen located directly next to a carbonyl (C=O).
What is the name of this functional group?

Amide.
What type of representation is shown below?
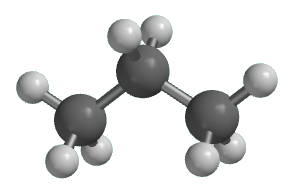
Ball and stick.
The compound 3-ethyl-2-methyl-4-propyldecane would contain how many TOTAL carbons?
16.
3-ethyl-2,3,5-trimethylhexane
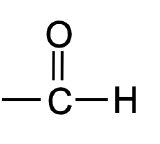
What is the name of this functional group?
Aldehyde.
What functional group contains a carbon triple bonded to another carbon?
Alkyne.
Which type of molecule would be considered to be saturated, an alkane or an alkene?
Alkane.
What is the correct CONDENSED structural formula for 4-ethyl-2,3-dimethylheptane?
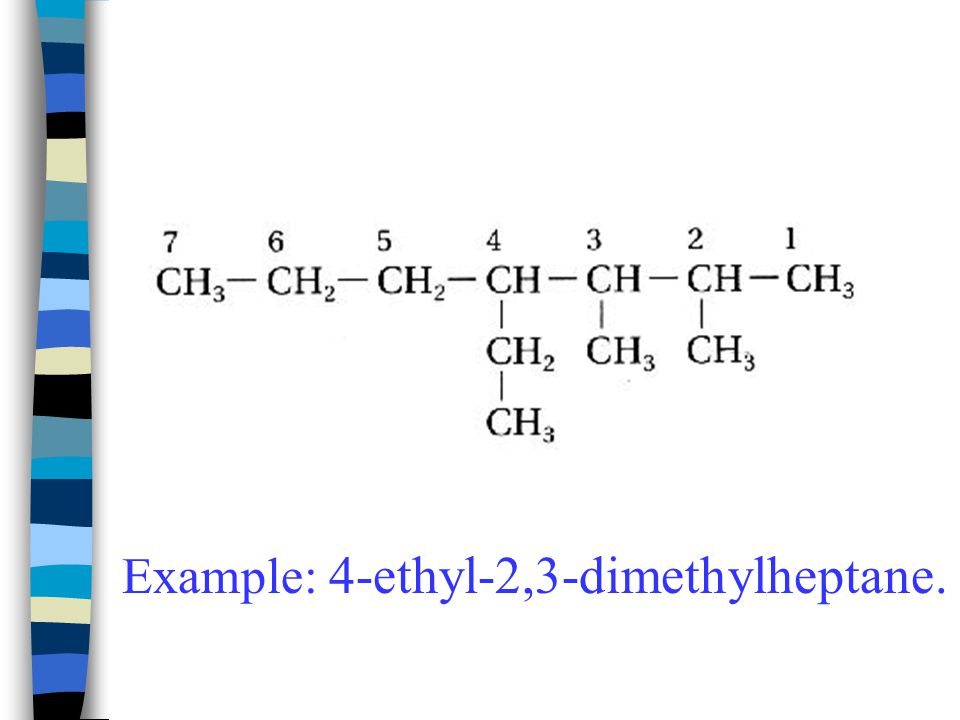
5-ethyl-4,5,6-trimethyldecane.
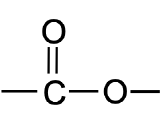
Draw the image of an ester.
Where is the carbon double bonded to an oxygen located in a ketone?
In the middle of your carbon chain.