PIONEER 2
PIONEER 3 & 4
The boxed warning for Ozempic® is with regard to which of the following? Select the best answer.
A. Risk of amputation
B. Risk of diabetic retinopathy
C. Risk of pancreatitis
D. Risk of thyroid C-cell tumors
D. Risk of thyroid C-cell tumors
In placebo-controlled trials, what were the most common adverse reactions with Ozempic® (excluding hypoglycemia, reported in at least 5% of patients treated with Ozempic® and more commonly than placebo)? Select all that apply.
A. Abdominal pain
B. Constipation
C. Decreased appetite
D. Diarrhea
E. Headache
F. Injection-site nodules
G. Nausea
H. Vomiting
A. Abdominal pain
B. Constipation
D. Diarrhea
G. Nausea
H. Vomiting
What were the weight reductions from baseline at week 56 in SUSTAIN 2 with Ozempic® and Januvia® (sitagliptin) (in combination with metformin and/or TZDs)? Select the best answer.
A. -3.8 kg (-8.4 lb) with Ozempic® 1 mg once weekly, -2.8 kg (-6.2 lb) with Ozempic® 0.5 mg once weekly, and -2.3 kg (-5.1 lb) with Januvia® 100 mg once daily
B. -4.1 kg (-9.0 lb) with Ozempic® 1 mg once weekly, -4.0 kg (-8.8 lb) with Ozempic® 0.5 mg once weekly, and -2.9 kg (-6.4 lb) with Januvia® 100 mg once daily
C. -4.8 kg (-10.6 lb) with Ozempic® 1 mg once weekly, -4.4 kg (-9.7 lb) with Ozempic® 0.5 mg once weekly, and -1.9 kg (-4.2 lb) with Januvia® 100 mg once daily
D. -5.5 kg (-12.1 lb) with Ozempic® 1 mg once weekly, -4.2 kg (-9.3 lb) with Ozempic® 0.5 mg once weekly, and -1.7 kg (-3.7 lb) with Januvia® 100 mg once daily
D. -5.5 kg (-12.1 lb) with Ozempic® 1 mg once weekly, -4.2 kg (-9.3 lb) with Ozempic® 0.5 mg once weekly, and -1.7 kg (-3.7 lb) with Januvia® 100 mg once daily
How does the semaglutide molecule compare between Rybelsus® and Ozempic®?
Identical semaglutide molecule is in Rybelsus® and in Ozempic®.
What was the primary endpoint in PIONEER 2?
What is mean change in A1C from baseline to 26 weeks
In PIONEER 3, Rybelsus® was used in combination with what background OAD(s)?
What is metformin with or without a sulfonylurea
What is Ozempic® indicated for? Select the best answer.
A. As an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus
B. As an adjunct to diet and exercise to improve glycemic control and manage weight in adults with type 2 diabetes mellitus
C. As an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus and to reduce the risk of major adverse CV events (CV death, nonfatal MI, or nonfatal stroke) in adults with type 2 diabetes mellitus and established CVD
D. To improve glycemic control in patients 1 year of age and older with diabetes mellitus
A. As an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus
What are the limitations of use with Ozempic®? Select all that apply.
A. Ozempic® has not been studied in patients with a history of pancreatitis. Consider other antidiabetic therapies in patients with a history of pancreatitis.
B. Ozempic® has not been studied in patients with severe gastrointestinal disease, including severe gastroparesis. The use of Ozempic® is not recommended in patients with pre-existing severe gastrointestinal disease.
C. Ozempic® is not a substitute for insulin. Ozempic® is not indicated for use in patients with type 1 diabetes mellitus or for the treatment of patients with diabetic ketoacidosis, as it would not be effective in these settings.
D. Use with caution in patients with liver disease.
A. Ozempic® has not been studied in patients with a history of pancreatitis. Consider other antidiabetic therapies in patients with a history of pancreatitis.
B. Ozempic® has not been studied in patients with severe gastrointestinal disease, including severe gastroparesis. The use of Ozempic® is not recommended in patients with pre-existing severe gastrointestinal disease.
C. Ozempic® is not a substitute for insulin. Ozempic® is not indicated for use in patients with type 1 diabetes mellitus or for the treatment of patients with diabetic ketoacidosis, as it would not be effective in these settings.
D. Use with caution in patients with liver disease.
What were the A1C reductions from baseline at week 56 in SUSTAIN 3 with Ozempic® and Bydureon® (exenatide ER) (in combination with metformin alone, metformin with an SU, or other diabetes medication)? Select the best answer.
A .-1.3% with Ozempic® 1 mg once weekly and -0.5% with Bydureon® 2 mg once weekly
B. -1.4% with Ozempic® 1 mg once weekly and -0.9% with Bydureon® 2 mg once weekly
C. -1.6% with Ozempic® 1 mg once weekly and -0.5% with Bydureon® 2 mg once weekly
D. -1.8% with Ozempic® 1 mg once weekly and -0.9% with Bydureon® 2 mg once weekly
B. -1.4% with Ozempic® 1 mg once weekly and -0.9% with Bydureon® 2 mg once weekly
What are the 2 “must wins” for Rybelsus®?
What is Own the Efficacy and Nail the Initiation
In PIONEER 2, Rybelsus® was used in combination with what background OAD?
What is Metformin
What were the observed A1C reductions at week 26 in PIONEER 3 for Rybelsus 7 mg, 14 mg, and Januvia 100 mg)?
What is -1.0 (7 mg), -1.3 (14 mg), vs. -0.8 (100 mg)
Which of the following is a once-weekly maintenance dose of Ozempic®? Select all that apply.
A. 0.25 mg
B. 0.5 mg
C. 1 mg
D. 2 mg
B. 0.5 mg
C. 1 mg
Which of the following is included in the diabetic retinopathy complications warning in the Ozempic® PI? Select all that apply.
A. In a 2-year trial involving patients with type 2 diabetes and high cardiovascular risk, more events of diabetic retinopathy complications occurred in patients treated with Ozempic® (3.0%) compared to placebo (1.8%).
B. The absolute risk increase for diabetic retinopathy complications was larger among patients with a history of diabetic retinopathy at baseline than among patients without a known history of diabetic retinopathy.
C. All patients who experienced a diabetic retinopathy event had never experienced retinopathy prior to the study.
D. In patients that did not have a documented history of retinopathy, there were no diabetic retinopathy events among patients taking Ozempic®.
E. Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy.
F. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy.
A. In a 2-year trial involving patients with type 2 diabetes and high cardiovascular risk, more events of diabetic retinopathy complications occurred in patients treated with Ozempic® (3.0%) compared to placebo (1.8%).
B. The absolute risk increase for diabetic retinopathy complications was larger among patients with a history of diabetic retinopathy at baseline than among patients without a known history of diabetic retinopathy.
What were the weight reductions from baseline at week 56 in SUSTAIN 3 with Ozempic® and Bydureon® (in combination with metformin alone, metformin with an SU, or other diabetes medication)? Select the best answer.
A. -4.0 kg (-8.8 lb) with Ozempic® 1 mg once weekly and -1.7 kg (-3.7 lb) with Bydureon® 2 mg once weekly
B. -4.8 kg (-10.6 lb) with Ozempic® 1 mg once weekly and -2.0 kg (-4.4 lb) with Bydureon® 2 mg once weekly
C. -5.6 kg (-12.3 lb) with Ozempic® 1 mg once weekly and -1.9 kg (-4.1 lb) with Bydureon® 2 mg once weekly
D. -6.4 kg (-14.1 lb) with Ozempic® 1 mg once weekly and -2.8 kg (-6.2 lb) with Bydureon® 2 mg once weekly
B. -4.8 kg (-10.6 lb) with Ozempic® 1 mg once weekly and -2.0 kg (-4.4 lb) with Bydureon® 2 mg once weekly
Why does the presence of food, excess liquid, or other oral medications in the stomach interfere with semaglutide absorption?
Semaglutide is primarily absorbed in the stomach after oral administration
Absorption is highly localized near the Rybelsus® tablet and the presence of food, excess liquid, or other oral medications can interfere with the absorption
Patients should be instructed to follow the Rybelsus® dosing instructions
What were the observed A1C reductions at week 26 in PIONEER 2?
What is -1.3 vs. -0.9
What were the observed weight reductions at week 26 in PIONEER 3 for Rybelsus 7 mg, 14 mg, and Januvia 100 mg)?
What is for -4.8 Rybelsus 7 mg, -6.8 Rybelsus 14 mg, and -1.3 Januvia 100 mg)
When used with Ozempic®, patients may require a lower dose of which medications to reduce the risk of hypoglycemia? Select all that apply.
A. Insulin
B. Metformin
C. SGLT-2 inhibitors
D. Sulfonylureas
E. Thiazolidinedione’s
A. Insulin
D. Sulfonylureas
How is Ozempic® administered? Select the best answer.
A. Once-daily oral tablet
B. Once-weekly oral tablet
C. Once-daily subcutaneous injection
D. Once-weekly subcutaneous injection
D. Once-weekly subcutaneous injection
What was the result relative to the primary composite endpoint of the SUSTAIN 6 study? Select the best answer.
A. No increased risk for MACE (CV death, nonfatal MI, or nonfatal stroke) was observed with Ozempic®.
B. Ozempic® significantly reduced the risk of major adverse CV events over 2 years, demonstrating superiority vs placebo for first occurrence of one of the following MACE events: CV death, nonfatal MI, or nonfatal stroke.
C. Ozempic® increased the risk of MACE, including CV death, nonfatal MI, or nonfatal stroke vs placebo.
D. Ozempic® resulted in significantly greater reductions in A1C at 2 years from baseline vs placebo.
A. No increased risk for MACE (CV death, nonfatal MI, or nonfatal stroke) was observed with Ozempic®.
Rybelsus® PI includes efficacy results from how many of the PIONEER trials
What is 7 (PIONEER 1, 2, 3, 4, 5, 6 and 8) 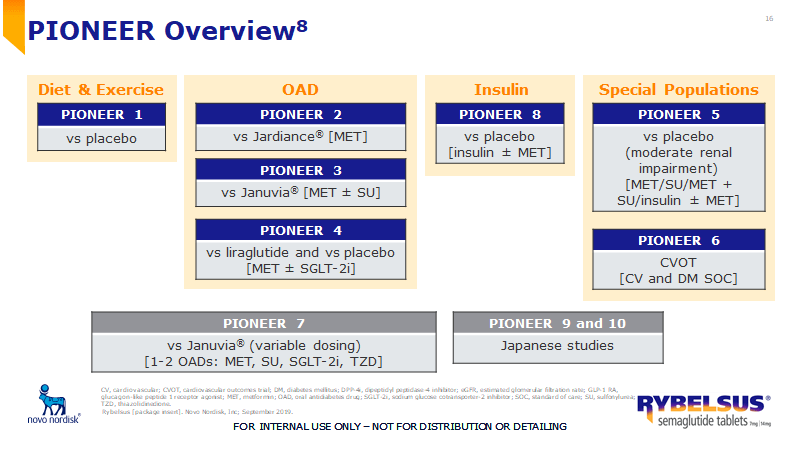
What were the observed weight reductions at week 26 in PIONEER 2?
What is -8.4 vs. -8.1
What % of patients achieved an A1C of <7% for Rybelsus 7 mg, 14 mg and Januvia 100 mg?
What is 44, 56, and 32%
What are some of the warnings and precautions with Ozempic®? Select all that apply.
A. Acute gallbladder disease
B. Diabetic retinopathy complications
C. Hypoglycemia with concomitant use of insulin secretagogue or insulin
D. Ketoacidosis
E. Risk of thyroid C-cell tumors
F. Pancreatitis
G. Acute kidney injury
B. Diabetic retinopathy complications
C. Hypoglycemia with concomitant use of insulin secretagogue or insulin
E. Risk of thyroid C-cell tumors
F. Pancreatitis
G. Acute kidney injury
What were the A1C reductions from baseline at week 56 in SUSTAIN 2 with Ozempic® and Januvia® (sitagliptin) (in combination with metformin and/or TZDs)? Select the best answer.
A. -1.2% with Ozempic® 1 mg once weekly, -1.6% with Ozempic® 0.5 mg once weekly, and -0.5% with Januvia® 100 mg once daily
B. -1.4% with Ozempic® 1 mg once weekly, -1.6% with Ozempic® 0.5 mg once weekly, and -1.1% with Januvia® 100 mg once daily
C. -1.5% with Ozempic® 1 mg once weekly, -1.3% with Ozempic® 0.5 mg once weekly, and -0.7% with Januvia® 100 mg once daily
D. -1.8% with Ozempic® 1 mg once weekly, -1.3% with Ozempic® 0.5 mg once weekly, and -1.1% with Januvia® 100 mg once daily
C. -1.5% with Ozempic® 1 mg once weekly, -1.3% with Ozempic® 0.5 mg once weekly, and -0.7% with Januvia® 100 mg once daily
Which of the following is part of the recommended storage conditions for the Ozempic® pen? Select all that apply.
A. After initial use of the Ozempic® pen, the pen can be stored for 8 weeks at controlled room temperature or in a refrigerator.
B. Prior to first use, Ozempic® should be stored in a refrigerator.
C. Ozempic® should not be frozen, prior to first use or after first use.
D. Ozempic® should be protected from excessive heat and sunlight.
E. The needle should always be removed and safely discarded after each injection, and Ozempic® should be stored without an injection needle attached.
F. The pen cap should remain on the pen when it is not in use
A. After initial use of the Ozempic® pen, the pen can be stored for 8 weeks at controlled room temperature or in a refrigerator.
B. Prior to first use, Ozempic® should be stored in a refrigerator.
C. Ozempic® should not be frozen, prior to first use or after first use.
D. Ozempic® should be protected from excessive heat and sunlight.
E. The needle should always be removed and safely discarded after each injection, and Ozempic® should be stored without an injection needle attached.
F. The pen cap should remain on the pen when it is not in use
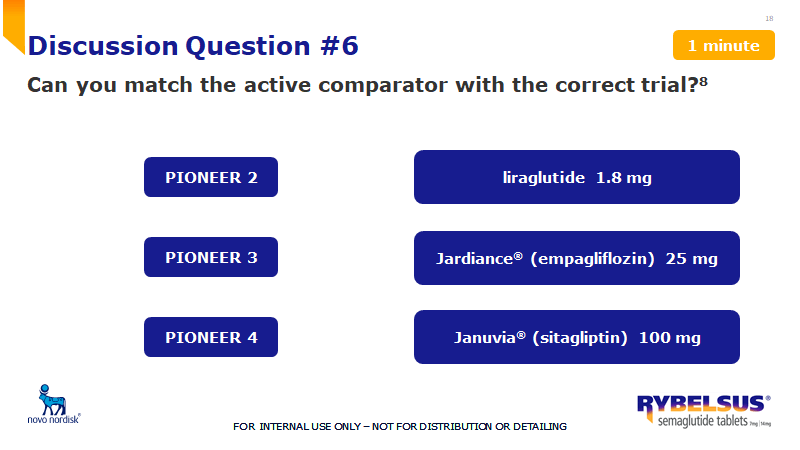
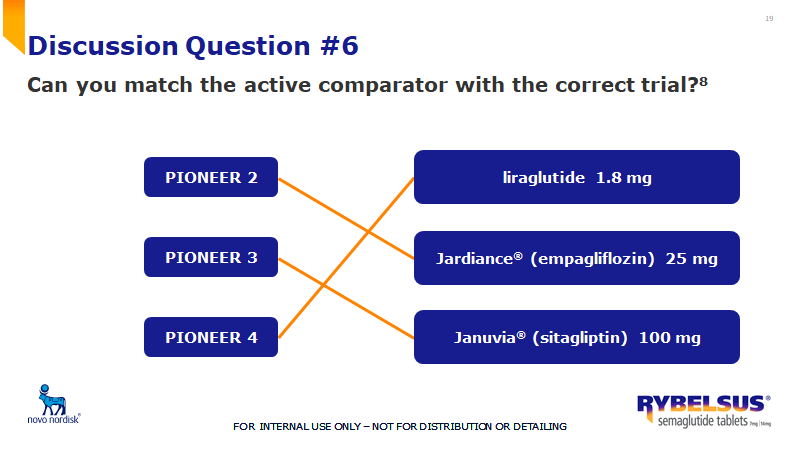
What % of patients achieved an A1C of <7% for Rybelsus 14 mg and Jardiance 25 mg?
What is 67% vs. 40%
At 26 weeks, Rybelsus® 14 mg resulted in statistically significant reductions in body weight vs liraglutide 1.8 mg and vs placebo. What were the results?
-6.8 Ozempic 1.8 mg vs -9.7 Rybelsus 14 mg