1. write the elements with their prefixes
2. the last element gets -ide
How do you name MOLECULAR compounds?
Atoms like to have 8 valence electrons around them.
what is the octet rule?
Bookkeeping system to keep track of electrons within a lewis structure.
The sum of all of this is zero.
The smaller this is, the better.
What is formal charge?
1 sigma and 2 pi bonds
triple bond
the measure of electrons in bonding and anti-bonding orbitals.
name of cation (charge) + base anion -ide
how do you name IONIC compounds?
These are the exceptions to the octet rule
Anything below the third row (expanded octets), radicals (odd number of electrons), B (wants 6), and H (wants 2).
bond forms from overlap of half-filled orbitals between 2 atoms
What is Valence bond theory?
has unpaired electrons, interacts with a magnetic field (magnetic)
what is paramagnetic?
This is the orbital with lower energy on a MO diagram.
What is a bonding orbital?
 the same lewis structures with a different position for delocalized electrons.
the same lewis structures with a different position for delocalized electrons.
What is resonance?
what is a nonpolar covalent bond?
Valence Shell Electron Pair Repulsion
(this uses lewis structures to predict the geometry of the molecule)
what is VESPR theory?
Has no unpaired electrons, not magnetic.
what does it mean to be diamagnetic?
Represented by a star in a MO diagram, this is the molecular orbital with higher energy
What is anti-bonding?
PbCl4
what is lead (IV) chloride?
∆EN= 0.5 -1.9
what is a polar covalent bond?
Bonds form when atomic orbitals combine to form molecular orbitals.
what is Molecular orbital theory?
atomic orbitals can mix together to yield hybrid orbitals.
What is orbital hybridization?
Name this: SrI2
what is strontium iodide?
magnesium bromide
MgBr2
∆EN = 2 or more
what is an ionic bond?
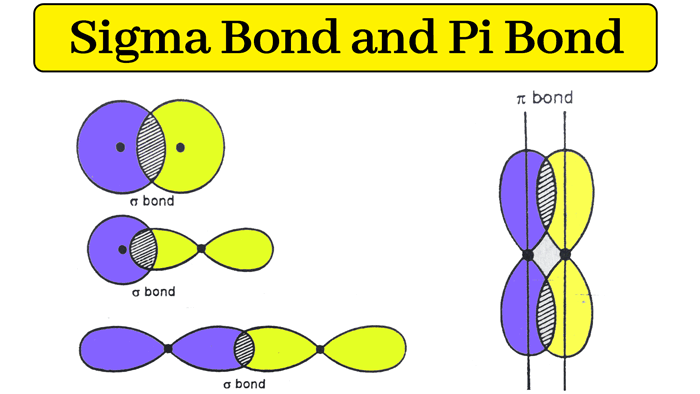
1-Bond where overlapping orbitals are pointed along the internuclear axis.
2- overlapping orbitals are peperndicular to internuclear axis
What is the difference between a sigma bond (σ) and a Pi bond?
What is the hybridization of Br in BrF3?
HINT: draw the lweis structure, determine the electron geometry, and then find the corresponding hybridization orbital.
sp3d
5 electron groups = trigonal bipyramidal= sp3d
What is the electron geometry and molecular geometry of PCl3?
eg: tetrahedral
mg:trigonal pyramidal
