Describes electron's orientation within an orbital. Possible values are +1/2 or -1/2.
What is the Spin quantum number (ms)?
Pauli exclusion principle
What says that no 2 electrons in an atom can have the same set of four quantum numbers?
IE: removing valence electrons
EA: adding valence electrons
Going from Be to B goes down instead of up
Ni to O decreases
What are the exceptions to the IE and EA trreand?
A bond that forms ionic compounds and involves the transfer of electrons.
What is an ionic bond?
Simplest whole number ratio of elements in a compound
what is empirical formula?
S orbital (spherical)
what is l=0 ?
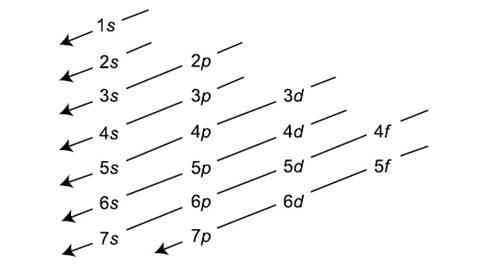
Electrons will fill orbitals from lowest to highest energy.
What is the Aufbau principle?
The average covalent bonding radius of an atom.
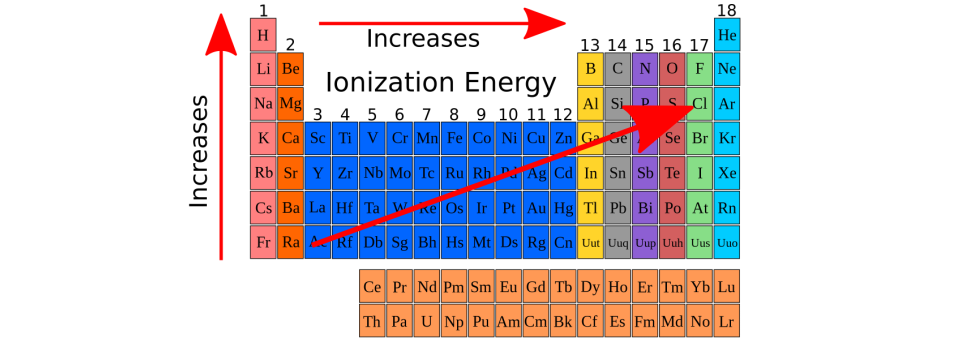
Decreases across a period, Increases down a group.
What is Atomic Radii?
A bond that forms molecules/molecular compounds and involves the sharing of electrons.
What is a covalent bond?
The number of each element in a compound.
What is molecular formula?
p orbital (two-lobed)
there are 3 p orbitals
what is l=1?
When filling orbitals of the SAME energy, place one electron in each orbital before paining up the electrons.
What is Hund's Rule?
The energy required to remove a valence electron from an atom.
Increases across a period, Decreases down a group.
What is ionization energy (IE)?
A covalent bond in which electrons are shared unequally.
What is a polar covalent bond?
calculate the mass percent of C in C2H4O2
40.022%
d orbital (4-lobed)
there are five d orbitals.
what is l=2?
These are exceptions to the Aufbau Principle.
what are Cu, Mo, Ag, Cr and Au?
Energy associated with the addition of an electron to an atom.
No trend down a group, Increases left to right.
What is Electron Affinity? (EA)
The more energy released means a larger EA (more negative value)
DAILY DOUBLE!!!
How many moles of KCl are in 0.556 L of a 2.3 M solution
1.3 mol
Find the empirical formula of a compound containing 0.672 g Co, 0.569 g As, and 0.486 g O.
Co3As2O8
1. find moles of each element
2. divide by smallest
3, put in whole numbers
f orbital (complex)
There are seven f orbitals
what is l=3?
Write out the electron configuration for calcium.
1s2 2s2 2p6 3s2 3p6 4s2
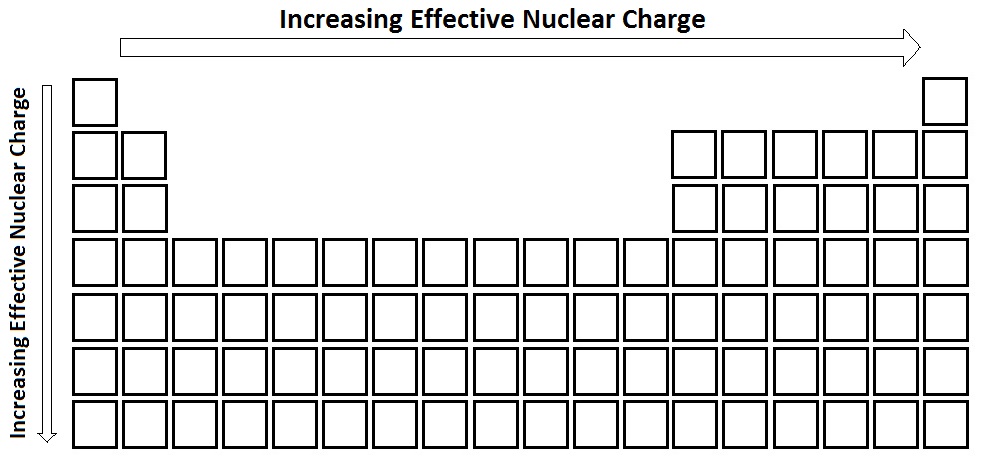
The charge that a valance electron "feels"
Increases left to right, Increases down a group
What is effective Nuclear charge?
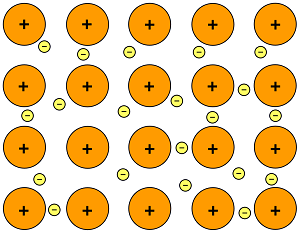
A bond in which electrons are shared around may positive nuclei in a metal sold.
what is a metallic bond?
Find the molar mass of phosphorus pentachloride.
PCl5
208.1 g/mol