The type of foil was used in Rutherford's experiment
What is gold?
The number of moles in 39.0983 g of K
What is 1?
Carbon - 14 mass number
What is 14?
The molar mass of NaCl? (hundreths place and correct units)
What is 58.44 g/mol?
The charge of an electron
The number of atoms in 1 mole
What is 6.022 x 1023 ?
The equation to find mass number
What is protons + neutrons?
The molar mass of Be(NO2)2 (hundredths place with correct units)
What is 101.83 g/mol?
The relative mass of a neutron
What is 1?
Who is Bohr?
An atom that looses 3 electrons has this charge
What is +3?
The number of neutrons in Magnesium-26
What are 14?
The average atomic mass of B (with correct units)
What is 10.811 amu?
Isotopes have different number of
What is neutrons?
The name of the scientist that proposed the idea of an atom.
Who is Democritus?
Ions that have a charge of -1
What is have gained an electron?
The mass number of an atom with 16 protons 16 electrons and 17 neutrons?
What is 33?
Calculate the average atomic mass of bromine. One isotope of bromine has an atomic mass of 78.92amu and a relative abundance of 50.69%. The other major isotope of bromine has an atomic mass of 80.92amu and a relative abundance of 49.31%.
What is 79.91 amu?
Ions have different numbers of
What are electrons?
Draw the model that was proposed by Earnest Rutherford
The number of moles in 38.04 g of H2O
What is 2?
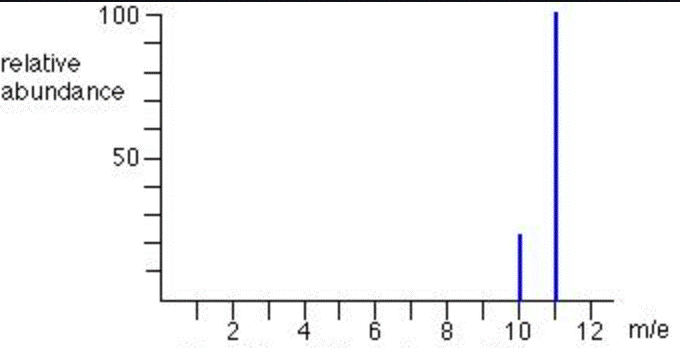 The element name of this mass spectrum would most likely be
The element name of this mass spectrum would most likely be
What is Boron?
The element copper has naturally occurring isotopes with mass numbers of 63 and 65.
The relative abundance and atomic masses are:
_____% for mass of 62.93 amu
30.8% for mass of 64.93 amu.
Find the missing percent and average atomic mass
What is 63.6 amu and 69.2%?