Make a prank call to a team members grandmother for these points.
MamMaw

What is Atomic Mass
This term is often used to describe an atom that has ionized by losing valence electrons, and thus results in a positively charged ion.
What is a cation.
This family is the most reactive family of metals on the periodic table?
What is the Alkali Metals
This element is used in the formation of today beverage cans.
What is Aluminum (Al)
This Russian chemist is credited with the creation of the first periodic table?
Who is Dmitri Mendeleev
On this side of the periodic table you can find the majority of the non-metals. Which of the nonmetals is not included on that side?
What is the right side and hydrogen
This charge will usually occur when Sodium ionizes
What is Na1+
Properties of this common family in the periodic table typically include; Shiny, strong and hard, ductile, malleable, good conductor, high melting points, and generally high densities.

What is the transition metals
This element is a very heavy "poor metal" and is often used as weight due to its extremely large density.
What is Lead (Pb)
Dmitri Mendeleev was the only chemist who left these in his rendition of the periodic table because he knew that other unknown elements existed.
What is gaps
These sections of the periodic table include all elements that contain similar properties as other elements.
What is a group. I will also accept family
This charge will usually occur when Sulfur ionizes
What is S2-
The elements in this family of the periodic table are often referred to as inert gasses. This is due to these atoms containing a filled octet
What is the Noble Gases
This noble gas is used as a shielding gas in GMAW (gas metal arc welding)
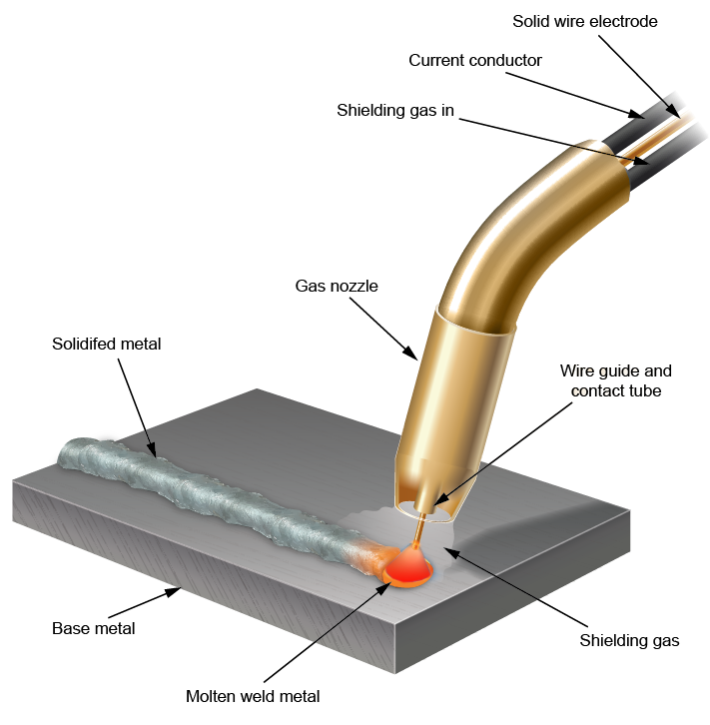
What is Argon (Ar)
Today's periodic table is now organized by increasing atomic number. This change was made by Henry Mosely. How was the periodic table arranged prior to this change?
What is by increasing atomic mass.
Using the organization of the periodic table. Correctly draw a Lewis Dot Diagram of the element Radon.
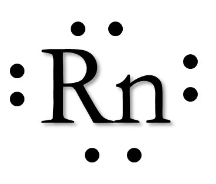
The Alkali metal family ionizes to produce a 1+ charge. Due to their ionization, they prefer to bond with this family in the periodic table to create many salt compounds?
What is the Halogens
This family in the periodic table contains the least amount of elements having only 4. Some properties of this family include high reactivity, elements in all three states at standard temperature and pressure, and readily form anions.
What is the Halogens
This element is used in the circuit boards of technology such as phones and computers mainly because of its ability to conduct electricity under certain conditions.
What is Silicon (Si)
What was one issue with some of the earliest periodic tables like the one attached?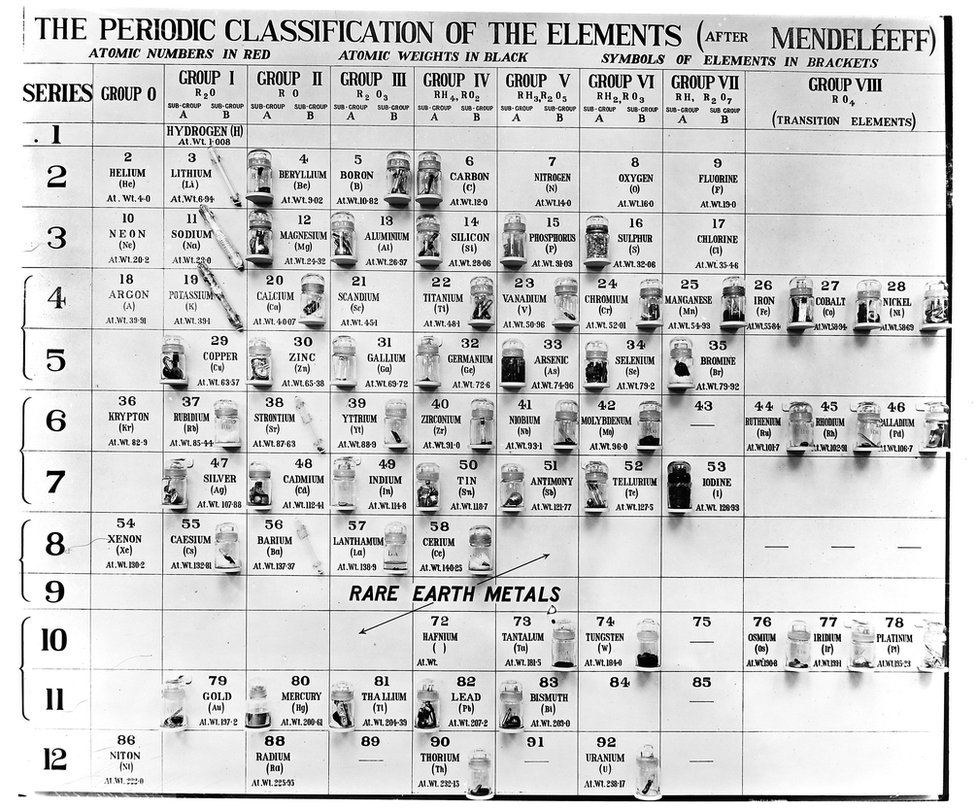
What is the order of the elements, the lack of elements, periods didn't equal the correct number of energy levels for every atom.
As you look inward on the periodic table, the reactivity of elements do what? Why is this?
What is decrease in reactivity due the need to gain/lose more electrons.
Will this element gain or lose electrons when ionizing? Idenrify an element it may pair with to achieve ionization and the ratio in which they will join.
What is lose and may join with any in groups 5, 6, or 7. Ratios will differ.
This family in the periodic table account for around 98% of the elements found in all living organisms.
What is the CHNOPS or other nonmetals
This element is found in the macromolecules that makeup all living organisms. It is often coined as the element of life.
What is Carbon (C)