
What does the 81 in this element represent?
atomic number
What group is neon in?
8A/noble gases
Are periods horizontal or vertical?
Horizontal
What is the atomic number equivalent to?
Number of protons/ electrons
Why are atoms of an element identified by the number of protons?
Each element has their own number of protons, no element is the same.
What does the 293 in this element represent?
Atomic weight
What group is silver in?
1B/ metals
What period is Antimony in?
Period 5
What is the mass number equivalent to?
Number of protons + number of neutrons
True or false? Alkali metals combine with Halogases easily.
True because they have 1 valence electron and Halogases have 7

What does the Ar in the element represent?
Chemical Symbol
What is group 7A also called?
Halogases
Period 7
What kind of charge do protons, electrons and neutrons have?
Positive, negative, and neutral
How do you divide an atom?
You can't, atoms can't be divided
 What does the S in this picture represent?
What does the S in this picture represent?
chemical name
What series is 57-71?
Lanthanide Series
What Element is in group 1 period 8A?
Helium
How many protons does silver have?
47 protons
Is promethium radioactive or artificially made?
Promethium is both
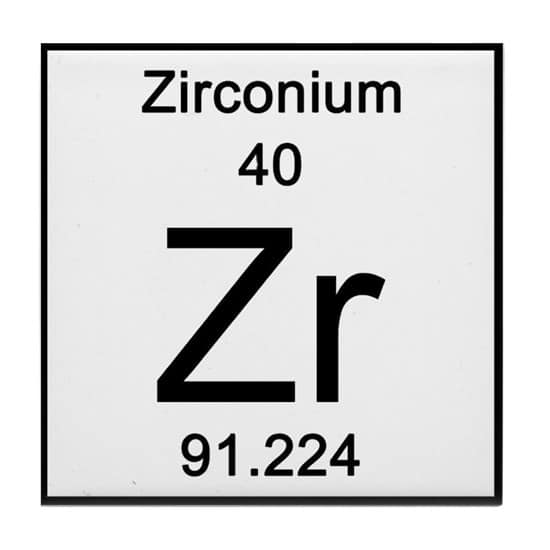
Using APEMAN, how many neutrons does the element Zirconium have?
A 40
P 40
E 40
_____
M 91
- A 40
_______
N 51
What series is elements 89-103 in?
The Actinide series.
What Element is in period 6 group 7A?
Astatine
How many protons, neutrons, and electrons does element Xe have?
54 protons, 77 neutrons, and 54 electrons.
How does the nucleus in the atom of one element differ from the nucleus of an atom of another element?
The nucleus in elements differ from each other because the number of protons and electrons are different.