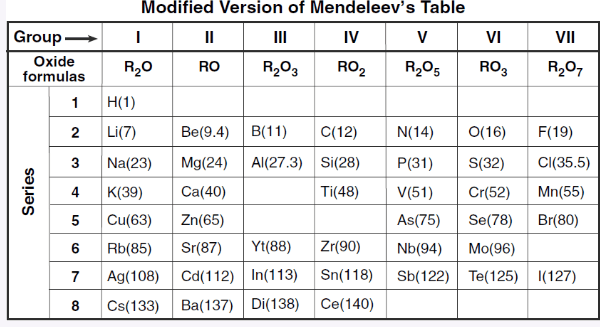
What class of element is found in Group III, Series 4?
Metallic
Which of the following will be a brittle solid that does not conduct heat or electricity?
Na Al Si S
S
What is the total number of electrons in a fluoride ion (F-) ?
10 electrons
Which element has the lower electronegativity?
Si or Cl?
Si has the lower electronegativity
Si
Metalloid
Which element would be likely to have an ion that produces a solution with a bright color?
Ca Cu Br
Cu
Transition metals often have ions that produce colorful solutions.

What is one property that Mendeleev used to identify and place the elements in this table?
Increasing atomic mass
Oxide formula
What is the trend in atomic structure as you move down a group?
There is an increase in the number of PELs and shielding as you move down a group.
What is the total number of electrons in a magnesium ion?
10 electrons
Which element will have the smallest radius?
C, N, O, F
F will have the smallest radius.
Al
Metal
What are two forms of an element with different molecular structures called?
Allotropes
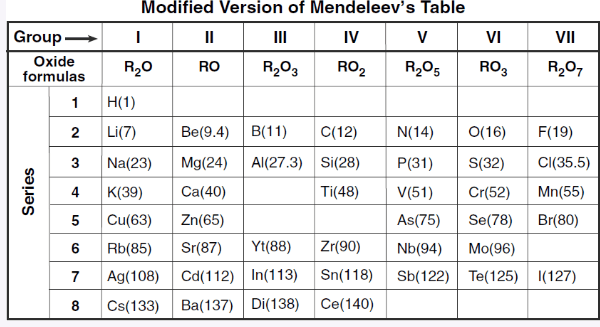
What is the most active metal in this table?
Cs
Cesium
What is the oxidation number for the metal in an oxide with the formula MO2?
+4
What is the Noble Gas that has the same configuration as the bromine ion?
Kr, Krypton
Which has the most metallic character?
C N O F
Carbon, C
Kr
Nonmetal
P has a larger radius and less nonmetallic character.
What is the oxidation number of elements in group VI?
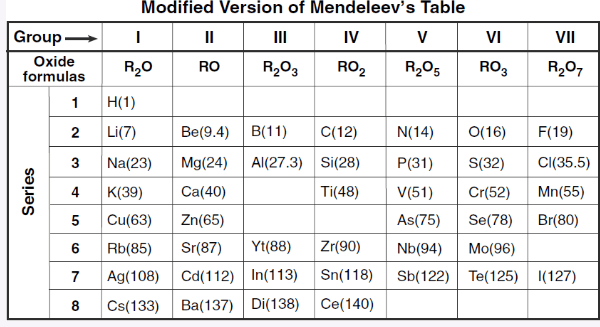
+6
Which of the following is an unreactive element?
P S Cl Ar
Ar: Group 18- Noble Gases- Unreactive
What is the Noble Gas that has the same electron configuration as the potassium (K) ion?
Ar, Argon
How do the atomic radius and ionization energy of Li compare to the atomic radius and ionization energy of N?
Li has a larger radius and lower ionization energy than N.
Co
Metal
What class of element exhibits both metallic and nonmetallic properties?
The metalloids
What group is not represented in this table and why?
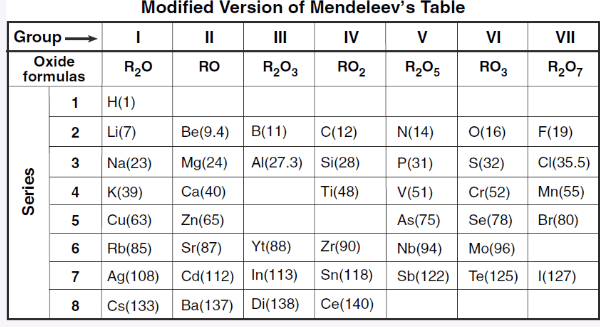
The table is based on reactivity, and group 18 elements (Noble Gases) do not react.
Group 18 is missing.
What is the trend in atomic structure across a period?
The #p+ increases (nuclear charge) which increases the nuclear attraction for electrons
(the number of energy levels (PELs) stays the same)
Is the the oxygen ion larger or smaller than an atom of oxygen?
Larger! Adding electrons causes the radius expand.
Which is the most active nonmetal?
P S Cl Ar
Cl, chlorine
C
Nonmetal
An element with seven valence electrons and an electronegativity greater than bromine could be
Kr Se I F
F, flourine