Cu
Copper
Ag
Silver
K
What is potassium
Al
aluminum
H
What is hydrogen
The Atomic Number is equal to the...
number of protons and electrons
Which element is a gas that reacts like an alkali metals
Hydrogen
__________ and _________ are the particles in the nucleus.
protons and neutrons
The noble gases are non reactive because
It has a full set of electrons.
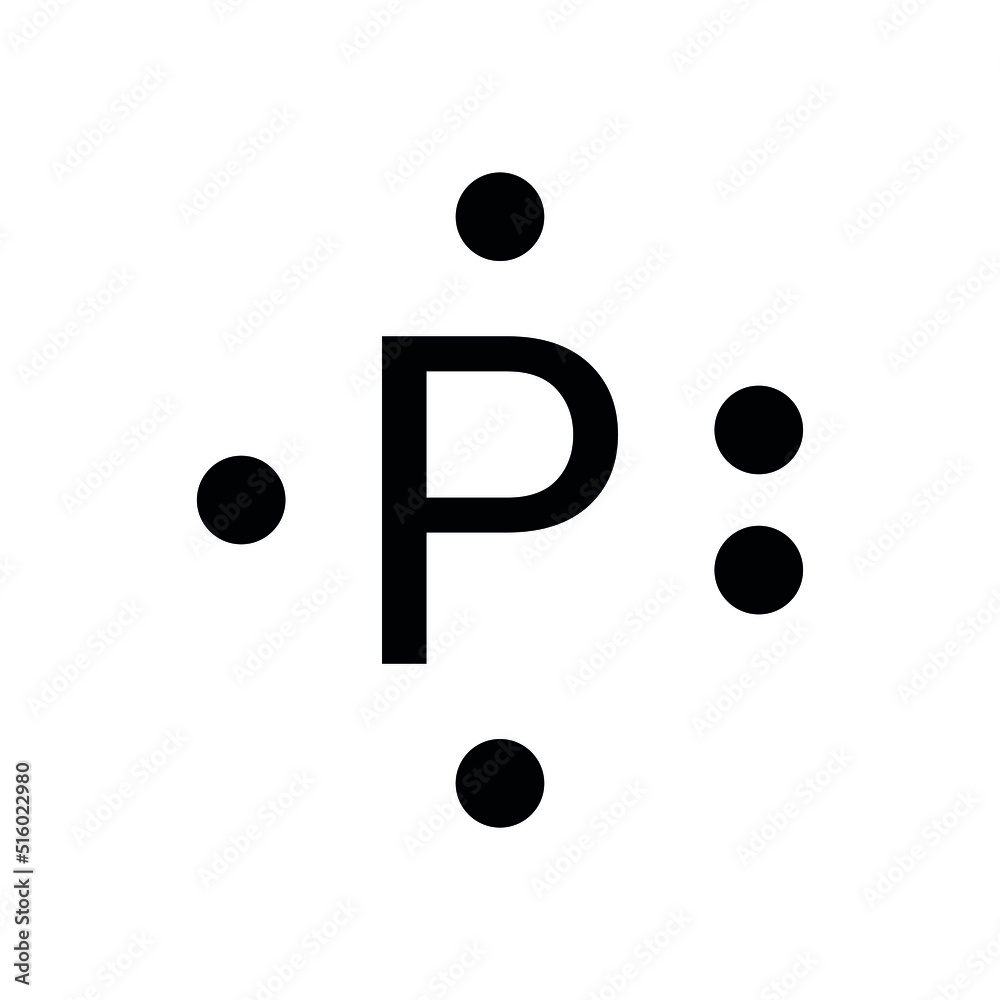
Go to the board and draw the Lewis Model of Phosphorous
The electrons in the outer most shell are known as?
Valence electrons
Identify the following elements S, P, O, N, C, H
Sulfur, Phosphorous, Oxygen, Nitrogen, Carbon, Hydrogen
Group 17 is called
Halogens
Group of elements that have characterisitics of metals and nonmetals.
Metalloids
Name the 2 liquids found in the periodic table
Mercury and Bromine
A horizontal row is called
Period
Group 18 elements are extremely nonreactive nonmetals known as ____________________
Noble gases
Group number of the Transition metals
3 to 12
Fluorine, Chlorine, Bromine, Iodine, Astatine, and Tennessine needs how many electrons to complete its last energy level.
One
Draw a Lewis Dot Diagram of Sodium and Chlorine. Give the ions formed.
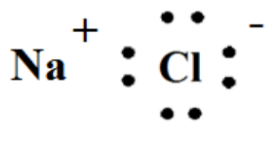
An isotope is an element that has a different number of...
neutrons
The most reactive group of the periodic table
Alkali Metals
The purpose of the zig zag line is to
Separate metals from nonmetals
Sodium and Chlorine joins together to form
table salt
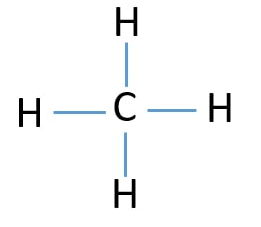
Covalent Bonds go to the board to draw a Lewis Dot Structure of CH4