I am in period 4 in the same group as this atom...
Kr
I have 4 valence electrons and 5 energy levels.
Sn
I am in the same period as Carbon but have 1 more proton.
N
I am the only metalloid in group 13
Boron
I have 3 electron shells and I do not react with other elements in nature.
Argon
What element is this Bohr Model showing?
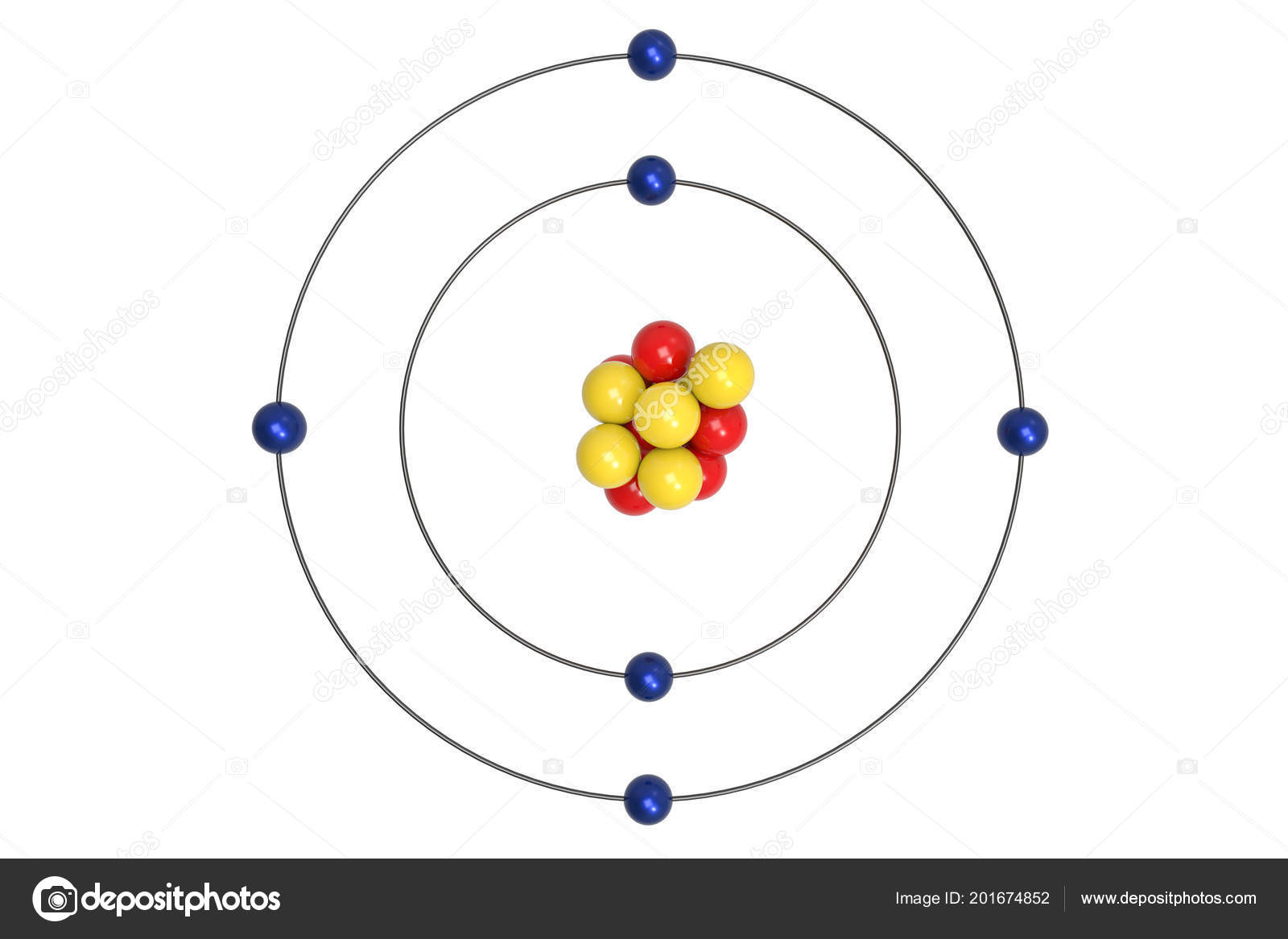
Carbon
Ca
I live in PERIOD 2, GROUP 17
Flourine
I am the only metalloid with 6 valence electrons
Te
I am an element used to make water in group 16.
O
Who would I react with in the same period as me?
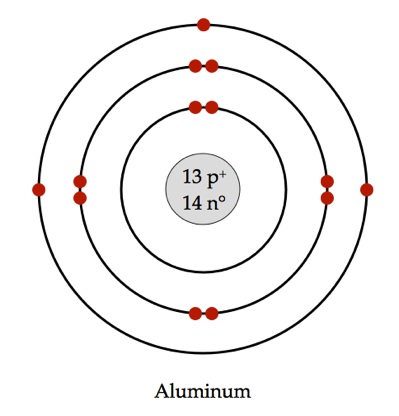
P
What is the only element with a full valence shell of 2 valence electrons?
He
I have the same valence electrons as Mg and I am in period 2.
Beryllium
I am a metal with 2 shells
Li
This metal is in the same group as Potassium (K), but is more reactive:
Is this Na or Rb?
Rb
What element am I?
Se
I am the first metal with 3 valence electrons.
Al
I have 2 shells and I am a noble gas (group 18).
Ne
I am the only metalloid with 3 energy levels
Si
I am Chlorine - who would I want to react with that is in the same period (3) as me?
Na
I am in period 5 and group 13. Who am I?
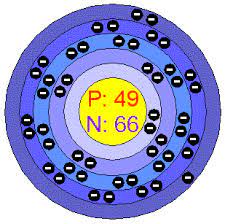
In
Rubidium (p = 37) reacts with which element in the same period?
I
I am an element in the 3rd period and the same family (group) as Bromine.
Cl
What is the only non-metal on the left side of the PT?
Hydrogen
Which element is more reactive?
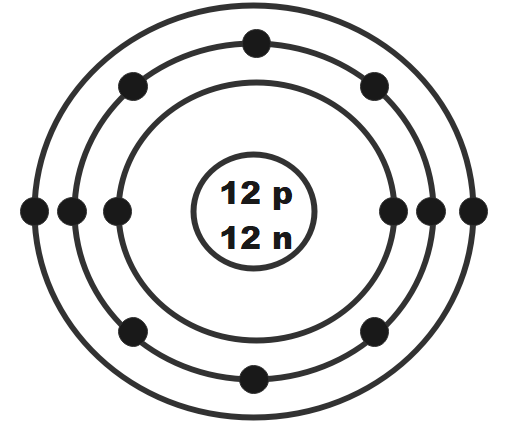

Mg
