What are the charges of each subatomic particle?
Proton, Neutron, Electron
Proton- positive
neutron- no charge
electron- negative
Which group are noble gases found?
Group 18

List the reactants and products in the following chemical equation.
NH3 --> N2 + H2Reactants: Products:
Reactants: NH3
Products: N2 + H2
Balance the following reaction
H2 + Cl2 --> HCl
H2 + Cl2 --> 2HCl
What is the name of the phase change when a gas changes into a liquid?
Condensation

An element has 24 protons, 23 neutrons, and 19 electrons. What element is this?
Chromium (Cr)

What period and group is carbon found in?
Period 2, Group 14
What does the coefficient 3 count in 3H2O?
The amount of molecules
Balance the following Reaction
H2 + O2 --> H2O
2H2 + O2 --> 2H2O
Which phase of matter has the least kinetic energy?
solid
Solids move very slow and kinetic energy is the energy of movement!
Write the isotopic notation for the element that has 8 protons, 9 neutrons, and 8 electrons.
For example, a carbon atom that has a mass of 13 is written in isotopic notation as carbon-13.
oxygen-17
Which elements have similar chemical properties?
a) lithium and beryllium
b) nickel and zinc
c) helium and neon
d) selenium and silicon
c) helium and neon
Elements in the same group have similar chemical properties!
Determine the type of chemical reaction below:
C + O2 --> CO2
Synthesis
C + O2 --> CO2
Carbon is adding to oxygen to make carbon dioxide.
Balance the following reaction
NaCl --> Na + Cl2
2NaCl --> 2Na + Cl2
What is the name of the physical property that measures the amount of matter that fits into a given volume?
Density

An element has 24 protons, 23 neutrons, and 19 electrons. Write the symbol and charge of this ion. (For example iron with a positive two chare is written Fe2+)
Cr5+
Chromium has 24 protons and 19 electrons. There are 5 more protons than electrons!
Which of these elements are malleable?
a) The element with 10 protons
b) The element in Period 4, group 15
c) The element with the smallest mass
d) The element with an atomic number of 29
d) The element with an atomic number of 29
Copper has an atomic number of 29! Copper is a metal and metals are malleable!
What law does balancing equations represent in the study of matter?
The law of conservation of mass. Matter is never created nor destroyed! The atoms rearrange themselves!
Balance the following equation:
CaCl2 + KBr --> KCl + CaBr2CaCl2 + 2KBr --> 2KCl + CaBr2
Which phase change requires a removal of thermal energy.
a) melting
b) sublimation
c) freezing
d) boiling
c) freezing
In order for something to freeze, it must lose thermal energy so the particles can move slower and come together!
Uranium-235 is highly unstable element that goes through alpha decay. This means the nucleus breaks apart by losing an alpha particle which is made up of two protons and two neutrons. What is the name of the new element formed when undergoes alpha decay.
For an extra 500, write it in isotopic notation.
thorium-231
This element is conductive, at room temperature its state of matter is a liquid.
Mercury

Which elements replaced each other in this single replacement reaction?
AlCl3 + 6K --> 3K3Cl + Al
Balance the following equation
C2H6 + O2 --> H2O + CO2
2C2H6 + 7O2 --> 6H2O + 4CO2
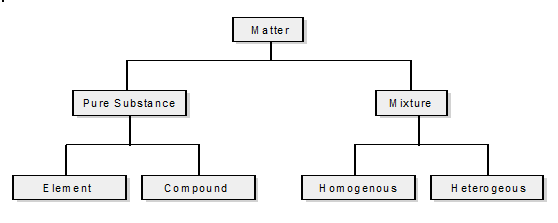
What are the two categories used to classify matter?
Pure substances and Mixtures