Which subatomic particle identifies elements?
Protons
What is the name of element with 16 protons?
Sulfur
Element in period 2 group 2
Be, Beryllium
What particle has a negative charge?
Electron
What element has 238 particles inside of the nucleus?
U, Uranium
Up to how many electrons can go on the last energy level?
8
Where are nonmetals located on the periodic table?
To the right of the stair case
What is the charge of the nucleus?
Positive
What is the name of the element with the element with 40 electrons?
Zirconium
Figure out the number of electrons on each energy level for NEON and draw it.
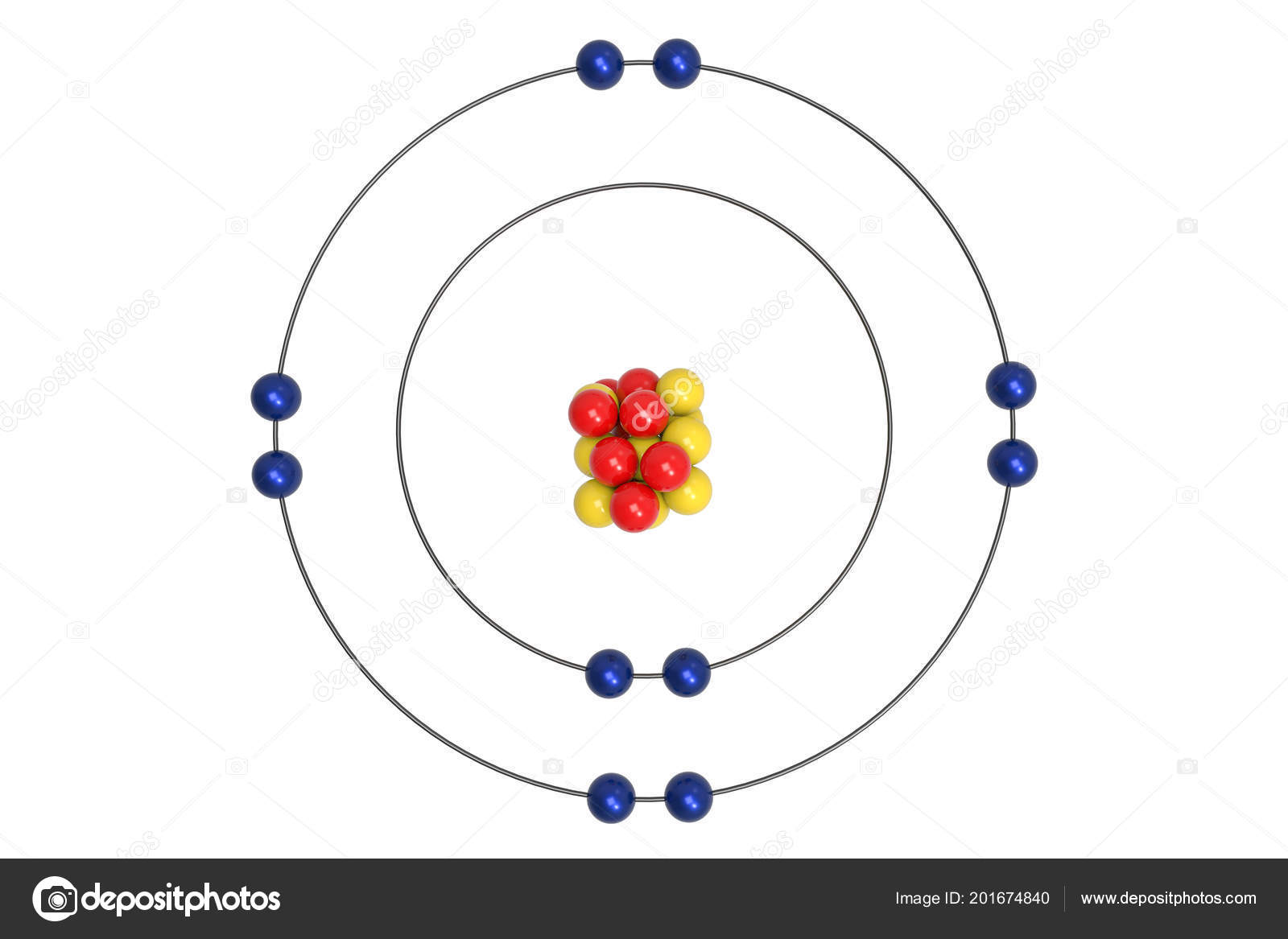
What determines the number of valence electrons in an atom?
Group
Which element has 42 particles outside of the nucleus?
Mo, Molybdenum
What element is a noble gas with 3 energy levels?
Argon
How many energy levels does Potassium have?
4
What determines the chemical properties of elements?
Valence electrons
What element has 3 energy levels and 7 valence electrons??
Chlorine
What element is a metalloid with 4 valence electrons?
Si, Silicon
What element has 5 valence electrons and 2 energy levels?
Nitrogen