Which subatomic particles make up the atomic mass?
What is the neutrons and protons.
Write the hyphen form of the isotope name for the information below: (Hyphen form: Name of the element - Atomic Mass)
10 protons
9 neutrons
10 electrons
What is:
Neon-19
Third period, eight valence electrons.
Argon
As you go from left to right in the metal side of the periodic table, what happen to the reactivity ? 
The reactivity decreases when going left to right on the periodic table.
The creator of the Periodic Table
Who is Dmitri Mendeleev
This number represents the number of protons in an atom.
What is the atomic number
How many neutrons does potassium-40 have?
What is 21.
Group 14 nonmetal.
Carbon
How many valence electrons does Group 2 have?
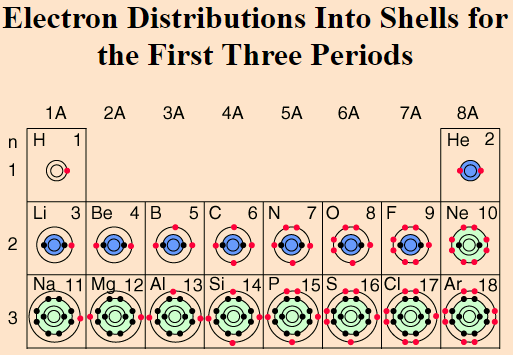
2 Valence electrons
This group of elements belongs to the family that has 7 valence electrons.
Who are the halogens.
Where are electrons located?
What is orbiting around the nucleus in the electron cloud
Write the isotope name in hyphen form for the following:
33 protons
41 neutrons
33 electrons
Arsenic-74
The element with the highest value for reactivity and electronegativity in Group 17. (Hint: Reactivity decreases as you go down in Group 17)
What is Fluorine.
How many valence electrons does Sodium have?

1 valence electrons
What do atoms of elements that are in the same group have in common?
They contain the same number of valence electrons.
A subatomic particle that has about the same mass as a proton.
a neutron.
Find the following for the isotope: Cobalt-59.
mass:
protons:
neutrons:
electrons:
What is:
mass: 59
protons: 27
neutrons: 32
electrons: 27
The halogen with electrons in the fifth energy level.
Iodine
Which element has the greatest reactivity in Group 1? (Hint: Reactivity increases as you go down in Group 1 -Alkali metals)

Francium.
These elements are dull, brittle and poor conductors of electricity.
What are non-metals (located on the right side)
Isotopes of an element contain different numbers of what subatomic particle?
What are neutrons.
Find the following for Iodine-126
mass:
protons:
neutrons:
electrons:
What is:
mass: 126
protons: 53
neutrons: 73
electrons: 53
An alkaline Earth metal that has a smaller atomic radius than Magnesium. (Hint: Remember the "Snow-man shape" The bigger number of period number, the more energy levels(orbits) the atom has , the bigger atomic radius it has)

What is Beryllium
Which family on the periodic table has no reactivity? Why?
The noble gases
Because they already have a full outermost ring.
How many groups are on the Periodic Table
What is 18