What is the maximum number of ELECTRONS the first ring/orbital shell can hold?
2 ELECTRONS
Russian chemist who is credited with creating the first widely recognized periodic table. (full name please)
Dmitri Mendeleev
A molecule is formed when two or more of these join together in a fixed ratio.
ATOMS
Often called table salt, this compound has the chemical formula NaCl.
SODIUM CHLORIDE
An element that has gained or lost an electron is referred to as
ion
They are typically shiny, ductile, malleable, and good conductors of heat and electricity.
METALS
What is the maximum number of ELECTRONS the second ring/orbital shell can hold?
8 ELECTRONS
These horizontal rows of elements in the periodic table are known by this name.
periods
This simplest diatomic molecule, composed of two of the same element, has the formula H₂.
HYDROGEN
A major greenhouse gas with the formula CO₂, it is also a byproduct of human respiration.
CARBON DIOXIDE
Found in Groups 3 through 12 on the periodic table, these metals often exhibit multiple oxidation states and form colored compounds.
TRANSITION METALS
These elements are generally poor conductors of heat and electricity and are often brittle in solid form.
NON-METALS
This subatomic particle has no charge.
NEUTRON
Elements in Group 2, including magnesium and calcium, are collectively known by this name.
alkaline earth metals
Why is the atomic mass not a whole number?

What are three physical properties of non-metals?
not shiny, not malleable (brittle), not ductile, poor conductors (good insulators), gain electrons, low density, low melting point
What category does the image represent?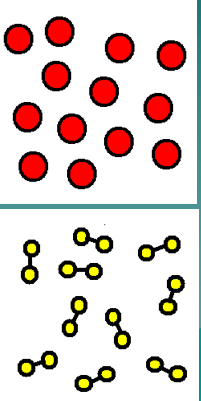
An Element and a Compound
This term describes a metal’s ability to be pulled or drawn into wires without breaking.
DUCTILE
In a neutral atom, the number of these _____________ is equal to the number of ___________.
PROTONS, ELECTRONS
What are the 3 parts of an atom
Protons, electrons, and neutrons
This molecule with the formula NH₃ is a pungent gas used in fertilizers and many cleaning products.
AMMONIA
Known commonly as baking soda, this compound (NaHCO₃) is used to help dough rise.
sodium bicarbonate
These metals are known for having just one of these in their outermost electron shell, making them highly reactive.
GROUP 1 METALS
Chlorine, which is a gas at room temperature, and sulfur, often found as a brittle solid, belong to this category of elements.
NON-METALS
This Greek philosopher proposed that matter is composed of tiny, indivisible units called “atomos.”
Democritus
This English scientist developed the first modern atomic theory in the early 1800s.
John Dalton
Found in the outermost shell of an atom, these electrons play a key role in chemical bonding and determine an element’s reactivity.
VALENCY ELECTRONS
What is the least reactive family on the Periodic Table?
Noble Gases
What is the least reactive family on the Periodic Table?
NOBLE GASES
This element has an unusual exception since it conducts electricity but is still classified as a non-metal. What is it?
CARBON