What is electronegativity? What is the most electronegative element?
Electronegativity is how well an element pulls shared electrons towards it in a bond. F is the most electronegative.
How many valence electrons does Helium have?
1s2
2 valence electrons
Draw a Bohr model for Ca2+
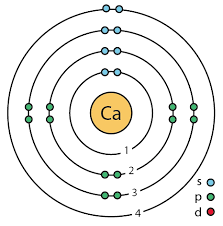
What element does this Bohr model represent?
Phosphorous
Which atom has a higher ionization energy? Why?
Lithium or Oxygen?
Oxygen because it is a smaller atom. If it is smaller, electrons are closer to the nucleus and harder to pull away. It also wants to gain electrons, not lose them.
How many valence and core electrons does Carbon have?
1s22s22p2
2 core electrons and 4 valence electrons
Draw a Bohr model for the common ion that Nitrogen forms. Write down what it's charge should be.
N3-
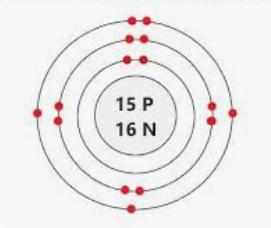
What element is represented by this Bohr model? What charge would its ion have?
Mg2+
Arrange the following from smallest to largest.
Rb, As, O and Cl
O, Cl, As, Rb
How many core and valence electrons does Argon have?
1s22s22p63s23p6
10 core electrons
8 valence electrons
Draw a Bohr model for the ion Potassium makes and give its charge.
K+
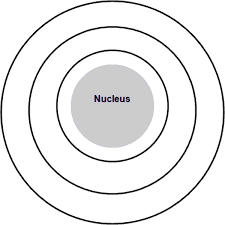
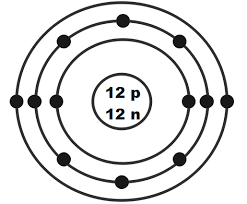
What element does this Bohr model represent? What charge would its ion have?
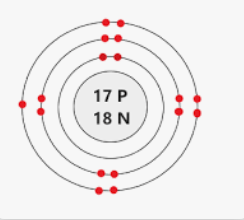
Cl-
What atom has the largest atomic radius? Why?
Francium because it has the most energy shells possible (7) but the fewest protons of any element in that period, meaning there is less of a pull from the nucleus.
How many core and valence electrons does Rubidium+ have? (This is a positively charged ion)
1s22s22p63s23p63d104s24p6
28 core electrons
8 valence electrons
Draw a Bohr model for the ion Sulfur forms and give its charge.
S2-
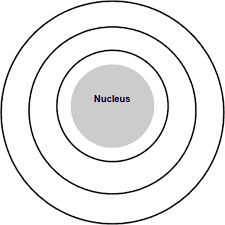
Name 3 elements this Bohr model could represent. Include charge if needed.
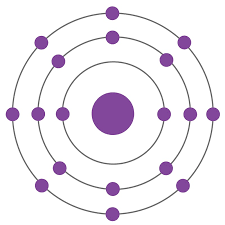
P3-
S2-
Cl-
Ar
K+
Ca2+
Which element should be the most reactive in Period 2 of the periodic table?
Based on our definition of reactivity, Lithium or Fluorine should be the most reactive, since they only need to gain or lose one electron.
How many core and valence electrons does Ra have?
[Rn]7s2
86 core electrons
2 valence electrons
Draw a Bohr model for the ion Beryllium forms and give it's charge. Include protons and neutrons.
Be2+
4 protons, 5 neutrons
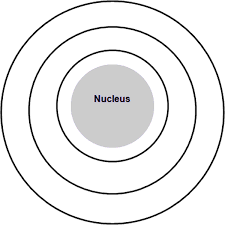
What are 3 elements this Bohr model could represent? Give charges if necessary.
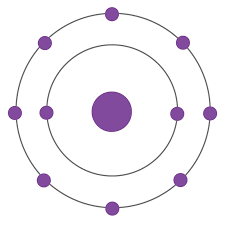
F-
O2-
N3-
Ne
Na+
Mg2+
Al3+