How many electrons does Argon have?
18
What period contains hydrogen and helium?
Period 1
How many electrons can an atom's first shell hold?
2
What kind of bond is formed between two ions of opposite charges?
ionic bond
What molecule's formula is H20?
Water
What is the atomic number of boron?
5
Which element is in Period 4 and Group 2?
Calcium
How many valence electrons does silicon have?
4
What kind of bond involves atoms sharing electrons?
What is a molecule made of? (Think in the simplest terms)
atoms
How many electrons are there in a phosphorus atom?
15
What is the name given to Group 2 of the periodic table?
Alkaline Earth Metals
How many electrons are in Magnesium's (Mg) second shell?
8
What kind of bonds did you represent with the dot structures you drew?
Covalent
Draw the dot structure for CH4
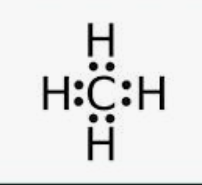
How many electrons are there in a beryllium ion (after a beryllium atom loses two electrons)?
2
What is the name of the group containing helium?
Noble Gases
How many valence electrons will you find in the outer shell of each Alkali Metal?
1
Looking at a periodic table, on which side (your right or left) of the periodic table are you most likely to find atoms that turn into negatively charged ions?
right
Draw a 3D picture of an alcohol molecule. You need to label each atom in the molecule.
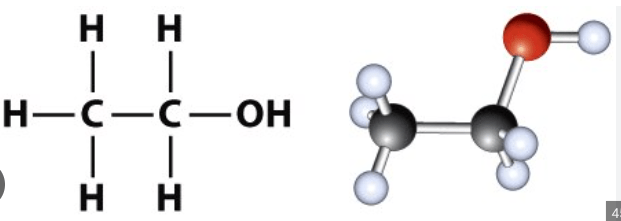
How many electrons are there in a chlorine ion (after a chlorine atom gains an electron)?
18
What is the name of the very reactive group that "wants" desperately to gain one electron?
Halogens
What is it about the electron arrangement of the Noble Gases that makes them so non-reactive?
Complete/Full outer shells of electrons.
What kind of a bond is lithium most likely to be part of?
ionic
How many electrons are shared between each C and O atom in a carbon dioxide molecule?
4