Radius
Which of the following elements have similar chemical properties?
A) Na, K, Ca
B) Mg, Ca, Ba
C) Ne, Ar, Kr, I
D) F, Cl, B
B) Mg, Ca, Ba
Atomic Radius ______ across a row and ______ down a group
Decreases; Increases
Ionization Energy ____ across a row and ____ down a group
Increases; Decreases
The force of attraction ___ across a row and ____ down a group
Increases; Decreases
____ is the most electronegative element
Fluorine
Choose the correct group or family that would have the highest first ionization energy
A) Halogens
B) Noble Gases
C) Alkali Metals
D) Alkaline Earth Metals
B) Noble Gases
The radius of cations generally ____ across a row.
Decrease
Which has the highest Ionization Energy:
A) Sn
B) Sb
C) As
D) Br
D) Br
Ca+2 or Ca has a greater force of attraction?
Ca+2
____ are the most reactive group of metals and ____ are the most reactive group of nonmetals.
Alkali Metals and Halogens
According to Coulomb's Law, as force increases, the charge _____; and the radius _______.
A) Decreases; Increases
B) Increases; Decreases
C) Decreases; Decreases
D) Increases; Increases
B) Increases; Decreases
Choose the ion with the largest radius:
Al+3 or N-3
N-3
Arrange the following in order of decreasing ionization energy:
S P N
N > S > P
Which of the following has the greatest force of attraction:
K+ Cl- Ar
Explain why noble gases don't have electronegativity values.
Electronegativity is ability to attract electrons in a bond. Noble gases are stable with a full valence shell, therefore do not bond or attract electrons.
In comparing the effective nuclear charge of Cl- to F-, which one would be higher? When becoming an anion, would the atomic radius increase or decrease?
F-; increase
Based on the following Ionization Energies, identify the atom with the largest atomic radius.
A - 494
B - 577
C - 418
D - 1255
Explain the difference in ionization between Mg and Al on the periodic table.
Mg - 738.1 kJ/mol
Al - 577.9 kJ/mol
Al requires less energy and is easier to remove since the p orbital is at a higher energy than the s orbital.
When comparing Coulombic attraction, identify the determining factor which has a greater effect on the force of attraction.
Distance to the outermost electron (Energy Level)
Identify the charges of the following elements:
K S Br Al
K+ S2- Br- Al3+
Justify why Oxygen has a lower first ionization energy than Nitrogen
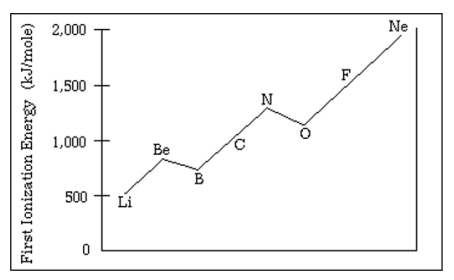
Oxygens last electron is in a paired orbital creating electron-electron repulsion making it easier to remove.
Arrange the following in order of increasing radius:
Mg P-3 Al Ca+2 Cl-
Ca+2 < Mg < Al < Cl- < P-3
Identify the following element in period 2 given its ionization energies:
1st: 899 kJ/mol
2nd: 1757 kJ/mol
3rd: 14849 kJ/mol
Beryllium
Arrange the following in order of increasing Coulombic Attraction:
Mg+2 P-3 As Ca+2 F-1
P-3 < F-1 < As < Ca+2 Mg+2
Place the following in order of increasing reactivity:
Li Al K Ca Mg Na
Al < Mg < ca < Li < Na< K