Identify the different states of matter
Solid, Liquid, Gas
How do we calculate energy (J)?
multiple the mass, specific heat capacity, and change in temperature of a substance in a calorimeter.
A physical change in a substance that converts from its liquid state to its gaseous state.
Evaporation
What 2 variables determine a substance's state of matter?
Temperature and pressure
How long has Demetrius been teaching?
12 years
Compare the particles in a solid and gas substance. How are they similar? How are they different?
Similar = there's some level of movement between particles in both states of matter
Different = the polar bonds in solids are much stronger than in gases and vice versa.
What is the unit of measurement for specific heat capacity?
J/g x oC
What's the difference between volatile and nonvolatile liquids?
Volatile = liquids that easily evaporate
Nonvolatile = liquids that do not easily evaporate
Which part of the graph represents the gas state?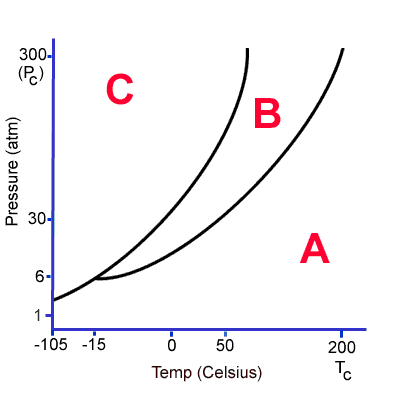
A
Which of the following names is the Demetrius's son's name?
-Carter
-Issa
-Kai
-Moussa
Kai

Give an example of an amorphous solid.
Glass, plastic, rubber
A block of aluminum occupies a volume of 15.0 mL and a mass of 40.5 g. What is its density?
2.7 g/mL
When the thermal energy is enough for molecules within the interior of a liquid to break free into the gas phase.
Boiling point
At what approximate temperature and pressure does the triple point for this substance exist?
Temp. = -15 oC
Pressure = 6 atm
Where did Demetrius grow up?
Virginia
What is matter?
Anything that takes up space and has mass.
Find the mass of 250.0 mL of benzene with a density of 0.8765 g/mL.
219.13 g
Why do ice cubes left in the freezer for too long become smaller?
Because the ice is going through the process of sublimation, turning directly into water vapor.
What does the triple point of a substance mean?
Where all three phases for a substance can occur simultaneously/at the same time.
What is Demetrius's favorite animal?
Orca
Identify the shape, volume, and compressibility properties of each state of matter.
Solid: shape and volume is definite and is incompressible
Liquid: shape is indefinite, volume is definite, and is incompressible
Gas: shape and volume is indefinite and is compressible
How many joules of heat are required to raise the temperature of 550 g of water from 12 oC to 18 oC? Water's specific heat capacity is 4.18 J/g x oC.
13,794 J
When water condenses, it can create steam or clouds. Condensation is an ________ process. How do we know?
Exothermic. It releases heat
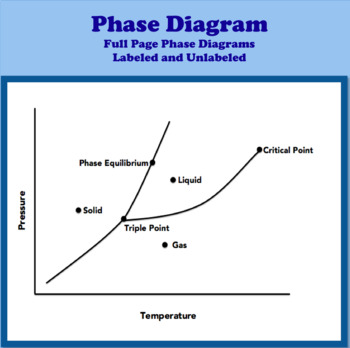
What does the line between solid and gas represent?
Where sublimation is occurring.
What country did Demetrius study abroad in college?
Australia