What is the approximate diameter of an atom?
1 x 10-10 m
What do we mean by "background radiation"?
Ionising radiation that is around us all the time, from a number of sources.
Give two dangers of using radioactive sources.
mutate DNA
mutate cells
kill cells
cause cancer
Give one disadvantage of using nuclear fuels to produce electricity.
Nuclear waste has to be disposed of.
Safety issues - if something goes wrong, it can go very wrong.
Public perception of danger
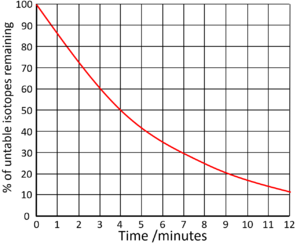 What is the half-life?
What is the half-life?
4 minutes
Who first proposed the idea of atoms having a nucleus?
Ernest Rutherford
What is the range of alpha particles in air?
A few cm
Give two safety precautions that a person who works with radioactive sources should take.
reduce exposure time
increase distance from the source
don't handle the source (use tongs)
work behind a screen
Give one advantage of using nuclear fuel instead of coal to produce electricity.
Nuclear fuel doesn't contribute to the greenhouse effect (No CO2 produced).
Much more energy produced (high energy density)
A scientist started with a mass of 60g of a radioactive substance.
After 30 days the mass was 7.5g. What is the half-life of the substance?
60 → 30 → 15 → 7.5
3 half-lifes
30 days ÷ 3 = 10 days
Draw a picture of a helium atom
How is a beta minus particle produced?
A neutron turns into a proton and emits a fast moving electron
Why are radioactive sources used as tracers in hospitals manufactured near to the hospital?
They have a short half-life, so need to be close, otherwise they would lose most of there radioactivity during transport.
What is the difference between nuclear fission and nuclear fusion?
Fission involves a large nucleus being split into 2 smaller nuclei.
Fusion joins together 2 smaller nuclei to make 1 large nucleus.
Complete this decay equation:
13153I → 0-1e + ?
13153I → 0-1e + 054Xe
Give the relative masses of:
a) proton
b) neutron
c) electron
a) 1
b) 1
c) 1/1835
What happens to the relative atomic mass and the atomic number when a nucleus undergoes alpha decay?
Mass number decrease by 4.
Atomic number decreases by 2.
Why is an alpha source used in a smoke alarm?
Alpha is very ionising, so will ionise air particles to complete the circuit in the smoke alarm
In a nuclear reactor, what is the role of the "moderator"?
It slows down neutrons, so they can be absorbed by the next atom
Polonium-208 undergoes alpha decay. Write a decay equation to show what is produced.
20884Po → 42He + 20482Pb
What do we mean by the term "isotope"?
An element that has the same number of protons, but a different number of neutrons
What happens to the relative atomic mass and the atomic number when a nucleus undergoes beta minus decay?
Mass number stays the same, atomic number increases by 1.
How are gamma rays produced in a PET scanner?
A positron is emitted from the source.
When it collides with an electron, both are annihilated.
Which produces 2 gamma rays, which travel in opposite directions
Why can nuclear fusion not happen at room temperature?
The nuclei do not have enough energy to overcome the electrostatic repulsion
Potassium-37 undergoes beta plus decay. Write a decay equation to show what is produced.
3719K → 0+1e + 3718Ar