Define a physical property and provide two examples
Characteristics that you can observe (qualitative) or measure (quantitative) without changing the identity of the matter
Examples include density, hardness, conductivity, magnetism, luster, mass, volume, and solubility.
What is a chemical property?
Characteristics of matter that can be observed as it changes to a different type of matter.
List four observable signs that are evidence of a chemical change.
he formation of light, heat, sound, bubbles, or a precipitate.
Bonus: formation of a new product
A scientist observes a substance's ability to react with acid. Which type of property is being investigated?
Chemical Property
What club does Ms. Le host in her room?
Disney Club
Are state changes an example of physical or chemical properties? Explain.
State Changes are an example of Physical Properties
Why? When matter changes states, it doesn't change what the matter is. Example: Ice cubes vs. Water vs. Water Vapor (all of these are made up of water molecules still)
What are the three types of chemical properties? (list all types to get the points)
Oxidation, Reactivity, Flammability
What is a precipitate, and what type of change does its formation indicate?
A precipitate is a solid substance that forms and separates out of a solution as the result of a chemical reaction. Its formation is an indicator that a chemical change has occurred.
During a lab experiment, two clear liquids are mixed, and a white solid substance forms and settles at the bottom of the beaker. What is this solid called?
a precipitate
What physical property is this showing? :max_bytes(150000):strip_icc()/antacid-tablet-dissolving-in-glass-of-water-84284196-58a30d095f9b58819ca7dd02.jpg)
Solubility
An apple turns brown after being left out on the counter for a couple hours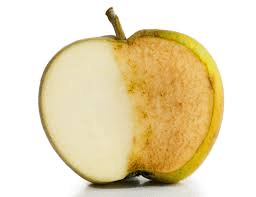
Oxidation
True or False
Chemical Changes can be reversed
False!
At least not easily...
A forests catches on fire and the wood of the trees is burned to ash. What is this an example of?
Flammability - Chemical Property
Which marine creature has three hearts and blue blood?
Octopus!
Which physical properties are size dependant? (must have all to get correct)
Mass, Volume, Density
Limestone (a mineral) comes into contact with hydrochloric acid and starts to form bubbles. What chemical property is this an example of?
Reactivity
True or False
phase changes are evidence of chemical changes
false!
Phase Changes only indicate a difference in the speed of the particles! The particles still stay the same, so no chemical changes have occured
Given the data, identify what the mystery substance is.
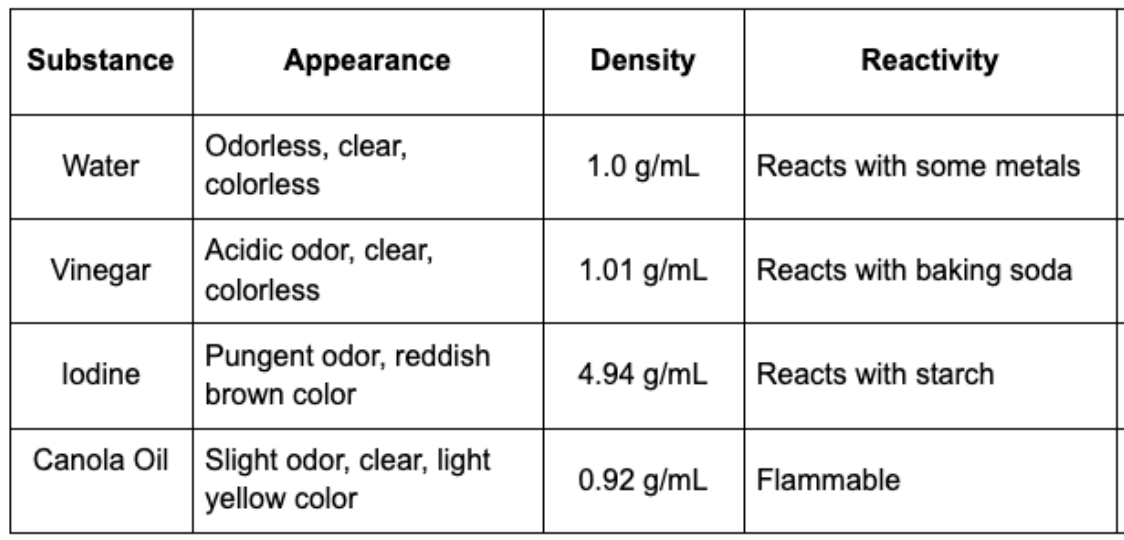
Mystery Substance observations:
- has a strong odor, red/brown, when in contact with starch, it forms bubbles, approx. 5g/ml
Iodine
Fill in the blanks:
Mass, Volume and Density are considered to be a _____ dependent property, which also makes it a _______________ property since you can measure it.
Mass, Volume and Density are considered to be a size dependent property, which also makes it a quantitative property since you can measure it.
You and your friends at the end of the year host a bonfire at the beach and decide to cook marshmallows over the fire. One of your marshmallows drops into the pit and burns to a crisp, eventually turning into ash.
What is this an example of?

Flammability
Why is color change not a reliable standalone indicator for distinguishing between a physical and a chemical change?
Color change is not a reliable indicator because both physical and chemical changes can cause it. Since it can occur in either type of change, one cannot rely only on color to determine what type of change has happened.
Ms. Le is feeling nauseous and the school nurse asks her to dissolve a tablet of Alka Seltzer into water and to drink it.
What is this an example of? What type of property is this?
Solubility - Physical Propery
Which letter is not found in the any of the US states' names?
