2 examples of physical properties.
color, mass, density, texture,
What is an element?
A type of atom.
12ft in inches
144inches
What is the formula AND units for density?
Density = Mass/Volume
g/cm3 or g/mL
What we call the last digit when we measure anything.
What is uncertain digit.
2 examples of chemical properties.
Flammability, rustability, reactivity, toxicity, ect.
What is the difference between a compound and a mixture?
Compounds are two or more elements BONDED together.
Mixtures are two or more types of PARTICLES mixed together but not bonded.
Number of miles in 2yds
0.00114miles or 1.14x10-3miles
What is the density if an object weighs 10.34g and takes up 22.2mL of space?
0.4658g/mL (correctly rounded counts)
The tool used to measure mass.
Scale or electronic balance
The difference between a chemical and physical change.
Physical doesn't change the chemical composition but a chemical change rearranges the molecular structure.
This is an example of a....
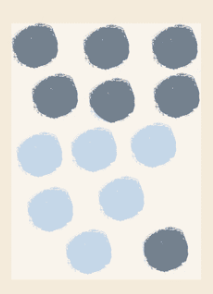
Heterogeneous mixture
5304mL into kL
0.005304kL or 5.304x10-3kL
A regular object measures 2.3cm x 34.5cm x 11.2cm and weighs 200g. What is it's density?
0.225g/cm3
Great for holding fluids, and estimating an amount, but very precise.
What is beaker or flask
Draw a particle model of a chemical change.
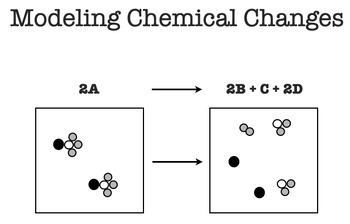
List an element, compound, and a homogenous mixture.
Answers will vary
Example: Sodium, Sodium chloride, and salt water.
The volume of 20g of iron, given if the density is 7.874 g/cm³
2.54cm3
Iron has a density of 7.874g/cm3. A iron nail was placed into 14.5mL of liquid and raised the level to 17.8mL. What is the mass of the nail?
25.98g
The US loves the imperial system but Chemistry likes to use this one.
What is the metric system
Why don't intensive properties change when you change the amount of the substance?
You're only changing the amount of mass/matter not their chemical or physical properties.
CO2 and O2 stirred together creates a BLANK of a BLANK and a BLANK.
Mixture of a compound and element.
How many grams are in 2.1tons of feathers?
1,905,087.95g
Draw a particle model of two objects with the same mass but different densities.

Something to this effect
What is the distance shown here? Include units.
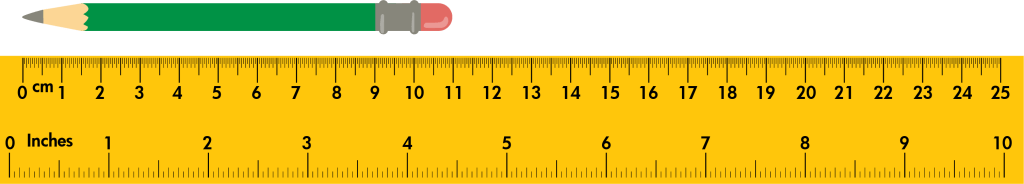
10.99cm plus or minus 0.1 (10.89 to 11.09)
Units and the correct number of digits must be included.