A state of matter that has no fixed shape or volume
Gas
You can "bite" into it leaving teeth marks.
What is gold?
Aluminum is a pure substance.

What is an element?
It is the amount of matter, or particles, in a substance.
What is mass?
A measure of the amount of matter present in a given volume of a substance.
What is density?
matter vs mass
mass is the amount of matter in an object
matter is anything that has mass and takes up space
It is best to use this unit of measurement for a liquid.
What is milliliter (mL)?
 What state of matter is this? and describe the particle relation,
What state of matter is this? and describe the particle relation,
What is solid? Each particle is attached to several others so it cannot move.
Mass is a form of this property.
What is physical?
The following measurements for a solid are as follows:
length x width x height = cm^3
What is the volume of a cube?
A characteristic of a substance that can be observed or measured without changing its composition
What is physical property?
Density describes the relationship between ____ and ____.
mass and volume
Which state of matter is this substance in? describe the particle relation.
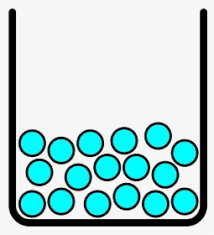
What is liquid? Particles are attached to one another and close but have the ability to slide past each other.
The more particles, the larger the object.
What is size-dependent?
True or False: the weight of an object is the same on Earth and on the moon.
What is false?
what do you use to observe physical properties of a substance?
your 5 senses (hearing, seeing, touching, tasting, and smelling)
scientific tools
The larger the shape, the more ______________ the substance has.
What is volume?
V = M/D
What is calculating volume?
M = D * V
What is calculating mass?
This chemical property is responsible for wood burning.

What is flammability?
How is the shape of solid matter different from the shape of liquids and gases?
Definite Shape. Particles form a set pattern.
Calculate the difference between the water level before and the water level after placing the object.
What is displacement?
A change in the physical substance but not the actual substance itself
Physical change
True or False: After going through a chemical change the atoms gain mass resulting in a new substance.
False. The atoms in the original substance rearrange to form a new substance.
This law states that the quantity of mass is conserved over time.
What is the law of conservation of matter?