A change in one or more physical properties of matter without any change in chemical properties is called a ______________ change.
physical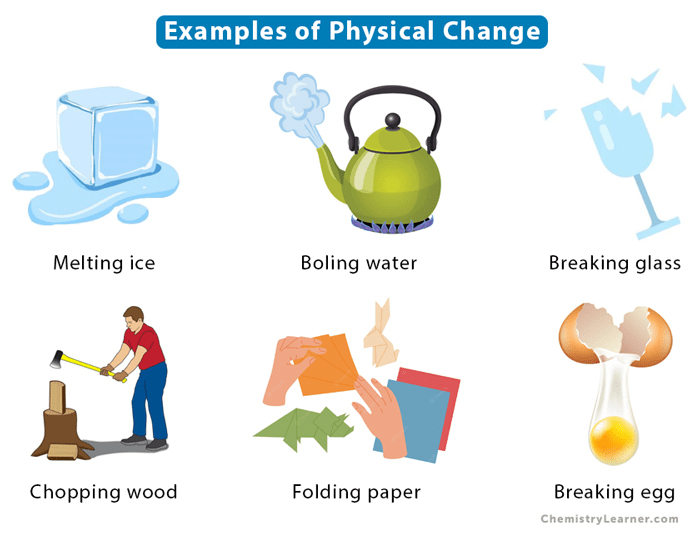
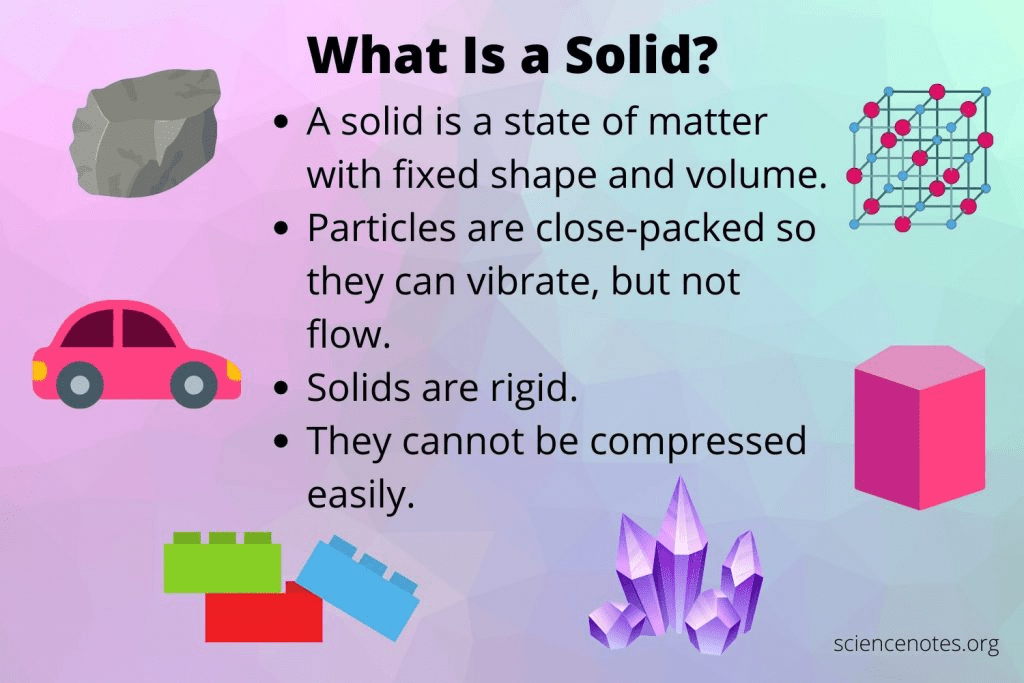
What state of matter has a definite shape and a definite volume?
A solid
In what phase change does matter change from a liquid to a solid as its particles lose energy until eventually the particle vibrate in a fixed position and are crowded closely together?
Freezing
The name of a particle with a negative charge.
What is an Electron?
True or False: salt completely dissolved in water is a homogeneous mixture.
True
____________ is defined as anything that has mass or volume.
Matter
What state of matter has a definite volume and takes the shape of its container?
A liquid

When melting occurs, are the particles gaining or losing energy?
When melting occurs, the particles are gaining energy. They absorb enough energy to partly overcome the force of attraction holding them together. This allows them to moveout of their fixed positions and slip over each other.

The name of the particle with a neutral charge.
What is a Neutron?
True or False: A diatomic molecule must be compound.
False
Abigail and Isaac, April students of the month, are investigating the properties of the same unknown liquid. Abby pours a large sample of the liquid into a beaker, and Isaac pours a small amount of the liquid into a small cup. which property of the liquid is the same for both Abby's sample and Isaac's sample? A. volume B. mass C. density D. shape.
C. Density
What state of matter has no definite shape and no definite volume?
A gas
What is the process in which a solid changes directly to a gas without going through the liquid state?
Sublimation
The 2D Model that represents electrons on circular energy levels around the nucleus.
What is the Bohr model?
True or False: In a colloid, particles will eventually settle.
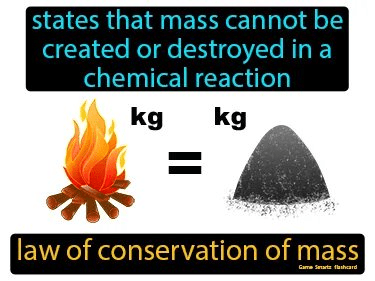
What is the law of conservation of mass?
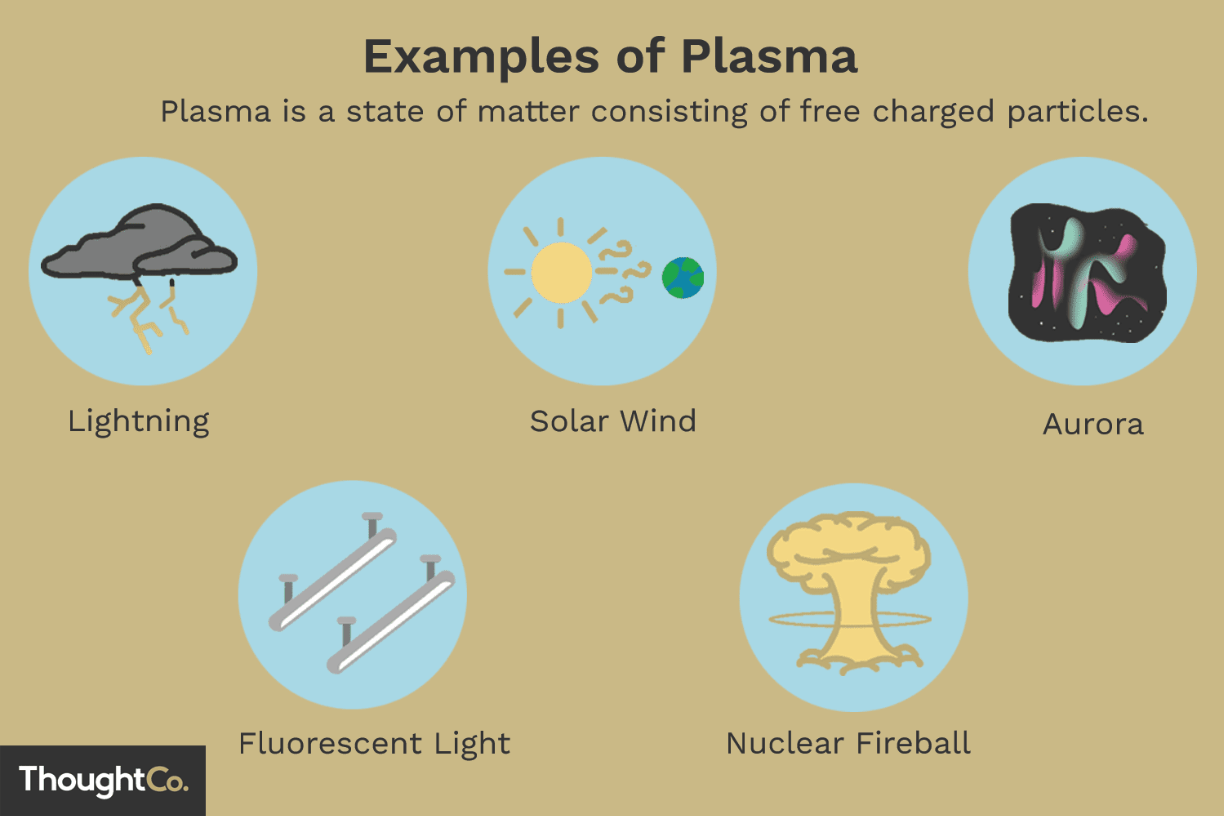
What state of matter lacks a fixed volume and a fixed shape and consists of charged particles?
Plasma
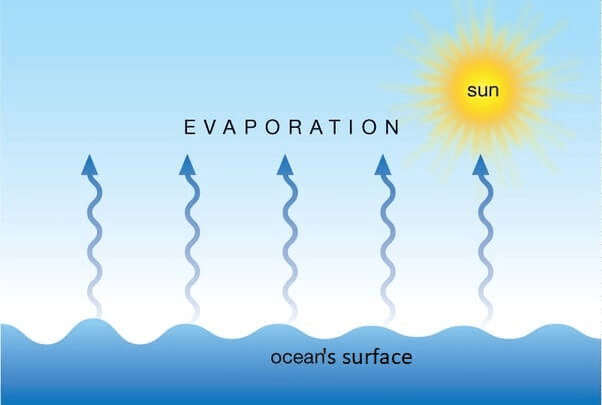
What is the process in which a liquid changes to a gas without becoming hot enough to boil?
Evaporation
What must happen for an electron to jump to a different energy level?
It must lose or gain energy.
The elemental symbol for Sodium.
What is Na?
A thermometer has graduations of 1 degree measuring temperature from 10-99 Celsius. How many significant digits should a measurement be taken have?
Three (e.g. 10.4 degrees Celsius)
The measure of the average kinetic energy of the particles in a substance.
What is temperature?
Absolute zero is -273 degrees Celsius or 0 degrees _____.
Kelvin
The name of the model that represents electrons in a cloud or orbital with an undefined location.
What is the Quantum Model?
What are pure substances?