What type of bond would you expect to be crated between an atom of Sodium (Na) and Bromine (Br)?
What charge would you expect an ion of aluminum to have?
What is 3+.
Atoms of the same element but with different number of neutrons?
What is an Isotope.
If a material has a definite volume but an indefinite shape, it would be classified as which state of matter?
What is a Liquid.
What is the appropriate metric unit for medicine?
What is mg.
What would you expect an atom of Iodine (I) to do to form a chemical bond?
What is gain 1 electron.
What type of charge does Metals have?
What is Positive charge (+).
If an atom has 16 protons, 13 neutrons, and 8 electrons, what is the mass number?
What is 29.
What is a type of plasma matter?
What is the sun.
If you were running or traveling a long distance, what unit of measurement would be used?
What type of bond would you expect to be created between Phosphorus (P) and Chlorine (Cl)?
What is Covalent Bond.
What happens to electrons if an atom has a 4- charge?
What is it will gain 4 electrons to make the atom stable.
Use this image to determine the number of neutrons in this atom?
What is 10.
What is an example of particles moving the slowest and becoming more compact?
Boiling water
liquid water
frozen water (ice)
What is frozen water (ice)
Order the following from larges to smallest. 5600mm, 0.008km, and 920cm?
What is 5600mm, 0.008km, 920cm
What type of bond transfer electrons and creates electricity?
What is an Ionic Bond.
On your periodic take Strontium (Sr), what charge does it have?
What is 2+.
Is this atom shown in a stable and neutral state, yes or no?
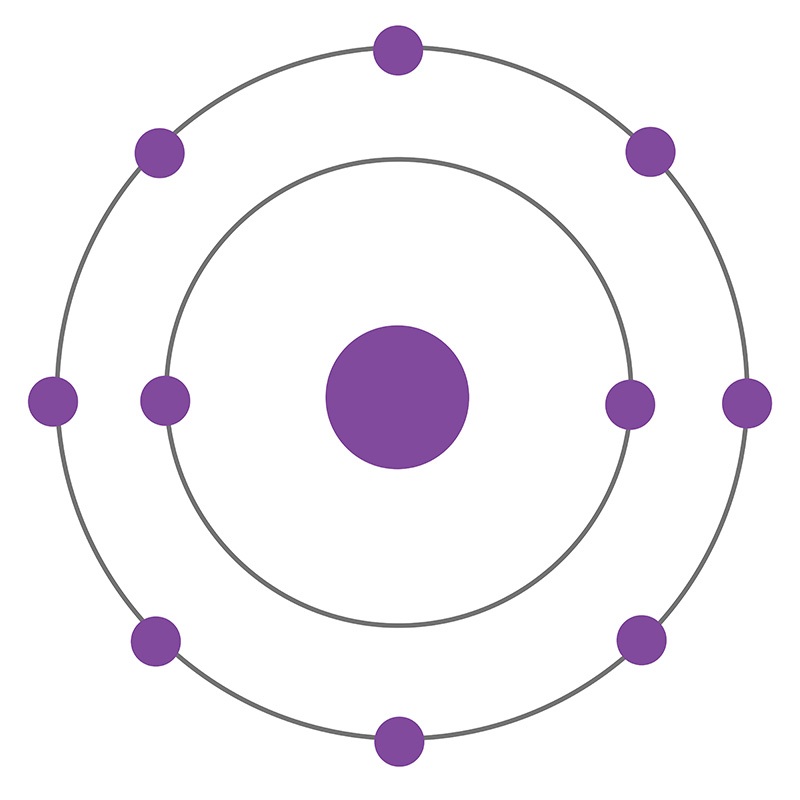
What is yes.
What is the process in which a gas becomes a liquid called?
What is condensation.
In 0.85kg, how may grams are there?
What is 850g.
Atoms with unstable outer energy levels can ________ electrons to obtain a stable outer energy level?
What is share or transfer electrons.
What is an Ion?
What is Gaining or losing of electrons.
Due to the location on the periodic table, how many valence electrons would you expect an atom of Lead (Pb) to have?
What is 4.
What is it when particles are far apart and move freely and at high-speeds?
What is a gas.
How many mm are in 10.9m?
What is 10,900mm.