The periodic table is arranged by increasing ________.
A. mass number
B. atomic number
C. groups
B. atomic number
True or False: A physical property changes the composition of matter.
False. It DOES NOT change the composition (what its made of).
Matter made of one kind of atom is called:
an element
Which of the following properties of matter never changes?
A. Mass
B. Volume
C. Density
C. Density (m/v)
The ________ number indicated the number of energy levels in a given atom.
A. Period
B. Group
C. Mass number
A. Period
A. Density
B. Flammability
C. Reactivity
A. Density
What are the four states of matter?
solid, liquid, gas, and plasma
The mass number is an average of the amount of protons and neutron in an atom. Where are the protons and neutrons located?
In the nucleus of the atom
A. Malleable
B. Luster
C. Melting Point
D. All of the above
D. All of the above
What has to happen to matter in order for it to change from a solid to a liquid.
Energy has to be removed.
Where are the metals located on the period table of elements? Give a direction (left/right/top/bottom)
To the left and middle
The physical property of matter that allows it to be drawn into wires is called ____________.
ductility
Identify the states of matter by the particles:
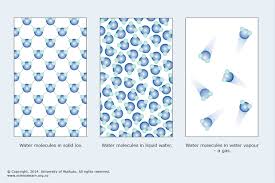
Solids, liquids, gases
Identify the following elements from their symbols:
Na, Ca, O
Sodium, Calcium, Oxygen
Which family of elements is generally stable?
The Noble Gases Group 18
Which of the following is an example of a physical property:
A. Over time, a copper penny turns green as it is exposed to air.
B. Sodium reacts easily with other elements
C. Copper conducts electricity.
C. Copper conducts electricity
Describe the process in which a solid turns directly into a gas. Give an example.
Sublimation. Dry Ice, certain air fresheners
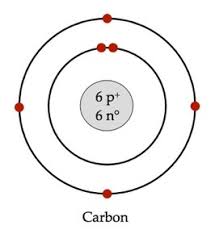
Draw a Bohr model of Carbon.
Atomic Number is 6
Mass Number is 12