In what year was the first Apple iPhone released?
2007
Compare decomposition and synthesis reactions.
Decomposition reactions break a single reactant down into two or more products. Synthesis reactions combine two or more reactants into a single product.
Compare endothermic and exothermic reactions.
Endothermic reactions absorb more energy than it gives off, while exothermic reactions release more energy than they absorb.
According to the collision model, what are the three requirements for a reaction to occur?
1. Must collide
2. Correct Alignment
3. Enough energy (activation energy)
List three signs that suggest a chemical change may have occurred.
- formation of a precipitate
- bubbles
- release of energy
- temperature change
- color change
- odor
- burning
- change in composition
How does a chemical equation model a chemical reaction?
A chemical equation shows the kinds and amounts of substances that enter into a chemical reaction as well as the kinds and amounts of products that are formed.
Which movie features a character named Lightning McQueen?
Cars
A reaction whose products have more energy than its reactants is a(n) ____________ reaction.
Endothermic or exothermic?
Endothermic
List 3 factors that alter reaction rates.
- changing temperature
- changing concentration of the reactants or products
- changing the surface area
- stirring
- adding a catalyst, enzyme, or inhibitor
How can you identify a combustion reaction?
Combustion reaction is when a substance reacts with oxygen and releases large amounts of energy (heat and light).
Determine if the following equation is balanced:
N_2+3H_2rarr2NH_3
balanced
Compare oxidation and reduction.
In oxidation, an element loses electrons. In reduction, and element gains electrons. During a redox reaction, one reactant is oxidized while another is reduced.
Think OIL RIG.
What is the most-watched YouTube video of all time?
Baby Shark Dance by Pinkfong
Why does changing the temperature change the equilibrium state?
When temperature changes, energy is added or removed from the system. The system will move in the direction of the endothermic process to reduce some of the energy that was added.
Does this graph represent an exothermic or endothermic reaction? 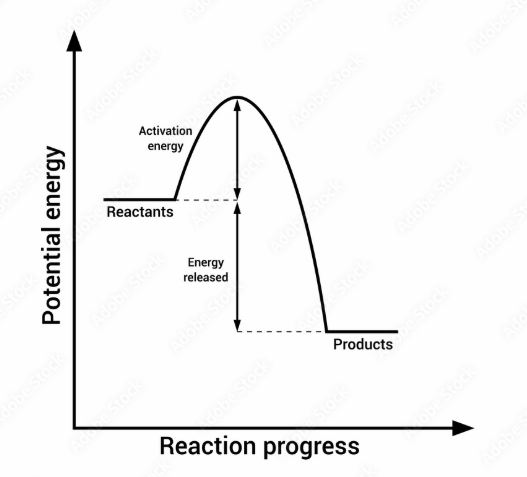
Exothermic (more energy is released than absorbed)
A chemist wanted to react 1 mol of methane (CH4) with chlorine gas (Cl2) to produce carbon tetrachloride (CCl4). How many moles of chlorine gas did he use for this reaction?
CH_4+4Cl_2rarr C Cl_4+4HCl
4 mol
What kind of reaction is shown below?
NaCl+AgNO_3 rarr NaNO_3+AgCl
Double-replacement
What must be true about the energy given off by an exothermic reaction and the energy remaining in the products according to the law of conservation of energy?
Together they must equal the energy originally in the reactants.
What is the most-ordered item at McDonald’s?
French Fries
Explain Le Chatelier's principle.
A system in equilibrium will adjust its equilibrium state to reduce the effect of any changes to the system.
Balance the following equation:
NH_3+O_2 rarr N_2O+H_2O
2NH_3+2O_2 rarr N_2O+3H_2O
The following reaction is a redox reaction. Which element goes through oxidation? Which goes through reduction?
Mg+S rarr MgS
Magnesium goes through oxidation (loses 2 electrons). Sulfur goes through reduction (gains 2 electrons).
How much energy is enough to cause a reaction to occur?
An amount equal to or more than the activation energy.
How does decreasing the concentration of lithium shift equilibrium?
4Li+O_2 hArr 2Li_2O
Shifts towards the reactants
Which athlete has the most medals in Olympic history
Michael Phelps