What must be done before forming the hypothesis?
"Gathering Information" or Research
solid to gas is called what?
Sublimation
The majority of an atom is made up of what?
empty space
is nitrogen gas (N2) a molecule, compound or both?
Molecule ONLY
If the mass of an object is 200 grams and the volume is 20 mL, what is the density?
10 g/mL
What is the tool used to measure the mass of an object?
Triple Beam Balance
The most common state of matter in the universe
plasma
The most abundant fundamental particle in the universe
Neutrinos

Classify this ^^
Heterogenous Mixture
Balance the following chemical equation:
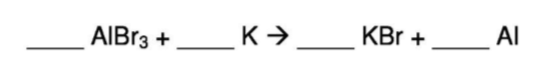
AlBr3 + 3K -----> 3KBr + Al
mass (m)
List two signs that tell you a chemical reaction has occurred
color change
formation of bubbles (foaming)
formation of precipitate (residue)
odor change
release of light
# of protons
or
atomic number
List two differences between metals and non-metals
Metals: lustrous, good conductors, malleable, ductile, mostly solid, used in construction, tools and jewelry, left side of periodic table, more reactive
Non-metals: dull, poor conductors, any state, brittle, used in biological systems and insulation, right side of periodic table, less reactive
If the density of an object is 300 g/mL and it takes up 3 mL of space, what is the mass of the object? (m)
900 g (grams)
What is the method by which you can measure the volume of a solid object?
"Water Displacement"
List two phase changes that involve a decrease in enthalpy
freezing, condensation, deposition, recombination
If an atom has more electrons than protons, what type of ion would it be considered?
Anion (-)
List at least two ways that substances can be physically separated
Handpicking (Sorting), Filtration, Magnetism, Distillation, Centrifugation
2C6H12O6 how many total atoms are in this compound?
48
Dr. Dunn is performing an experiment to see how music affects a student's level of focus. He chooses 10 different music genres and gives students an assignment. What would the independent variable of this experiment be?
Genre of music
List one difference between "boiling" and "evaporation"
Boiling: occurs throughout the liquid at a specific temperature (boiling point)
Evaporation: occurs at the surface of the liquid gradually (temperature range)
What fundamental particles make up protons in the nucleus? (be specific!!!)
How would you classify air?
Homogenous Mixture
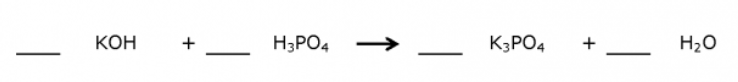 Balance the Equation
Balance the Equation
Dr. Dunn is performing an experiment to see how music affects a student's level of focus. He chooses 10 different music genres and gives students an assignment. What would the "control" of this experiment be?
No Music
Describe the law of conservation of mass
"Matter is neither created nor destroyed during a chemical change"
or
"The mass of the products is equal to the mass of the reactants"
List the 4 fundamental forces in order of increasing strength
gravitational force < weak nuclear force < electromagnetic force < strong nuclear force
Give the chemical symbol for the only metal that exists in the liquid state
Hg (Mercury)
A graduated cylinder is filled to the 20 mL mark with water. A 30 kg object is dropped into the cylinder and the water rises to the 60 mL mark. What is the density of the object?
0.75 g/mL