Chapter 10 Nuclear Chemistry
Chapter 6 Chemical Bonding
Chapters 4 and 5 Periodic Table
Chapter 7 and 2: Balancing and Rate of Reaction
Chapter 3: Phases of Matter
100
This is the definition of an isotope
What is an atom that has the same number of protons, but different numbers of neutrons?
100
This is the difference between the atomic number and mass number in the periodic table
The atomic number is the number of protons;
The mass number is the number of protons and neutrons
100
This is the difference between a cation and an anion
What is a cation is a positively charged ion that has lost electrons; an anion is a negatively charged ion that gained electrons
100
This is the substances that are formed during a chemical reaction
What is a product?
100
This is a state of matter in which the material has a definite volume, but not a definite shape (fits the shape of the container)
What is a liquid?
200
This is process in which an unstable atomic nucleus emits charged particles and/or energy
What is radioactivity?
200
This is the location of protons, neutrons, and electrons in an atom
Protons and neutrons are found in the center of the atom in the nucleus; electrons are found outside the nucleus in an electron cloud.
200
This is the difference between ionic and covalent bonding
What is
Ionic bonding occurs when electrons are transferred from a metal to a nonmetal;
in covalent bonding electrons are shared between nonmetals
200
Identify the coefficients and subscripts in this equation:


Coefficients: 2 in Na2O2 and 4 in NaOH
subscript:numbers in N2O2, H2O, O2
200
Phase change from solid to gas
What is sublimation?
300
This is the definition of a half life
What is the amount of time it takes for half a radioactive sample to decay?
300
This is the atomic number and mass number of chlorine


What is 17 and 35?
300
Which groups are most likely to form cations?
Which groups are most likely to form anion?
What is Groups 1A-3A form cations and groups 5A-7A form cations?
300
These are pure substances
What are elements and compounds?
300
One example of a phase change that creates a more orderly arrangement of particles?
What is freezing, deposition, OR condensation?
400
Identify the atomic and mass number of this isotope:
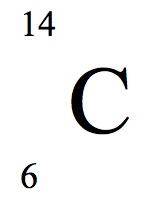
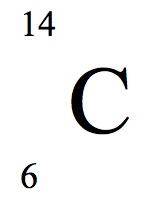
The atomic number is 6.
The mass number is 14.
400
This is the number of valence electrons for lithium 

What is 1? (Group 1A)
400
This is the difference between a polar covalent and a nonpolar covalent molecule
What is in a polar covalent molecule electrons are shared unequally while in a nonpolar covalent bond electrons are shared equally?
400
Determine if the following equation is balanced (if not, explain why)


No, the oxygen atoms are not balanced on the left (reactant side)
400
This is a phase change in which energy is ABSORBED (hint: bonds are broken)
What is melting, sublimation, vaporization?
500
This is the type of decay in this equation:

What is alpha decay?
500
This is the correct name for this ionic compound:
AlCl3
and the correct name for this covalent compound:
P2F4
What is
aluminum chloride
and
diphosphorous tetrafluoride?
500
This is the formula for calcium permanganate
What is Ca(MnO4)2
500
These are four ways to increase the reaction rate
What is surface area (break up the reactant into smaller pieces), increase the rate of stirring, and use a catalyst?
500
This is what happens to potential and kinetic energy during a phase change
What is kinetic energy remains constant and potential energy increases?