A change in one or more physical properties of matter without any change in chemical properties is called a ______________ change.
physical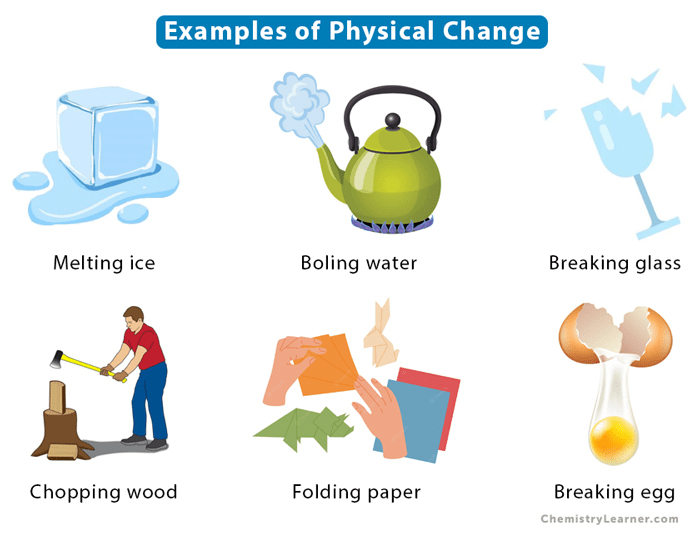
What state of matter has a definite shape and a definite volume?
A solid
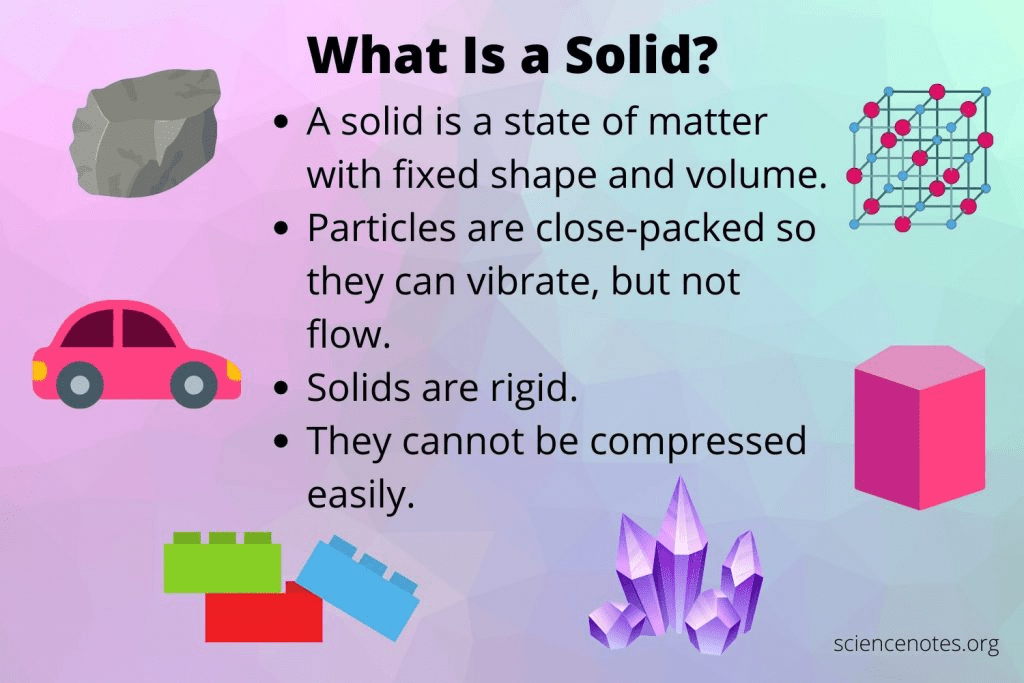
In what phase change does matter change from a liquid to a solid as its particles lose energy until eventually the particle vibrate in a fixed position and are crowded closely together?
Freezing
Name the three parts of an atom and give their charges.
Protons have a positive charge.
Electrons have a negative charge.
Neutrons have no charge.
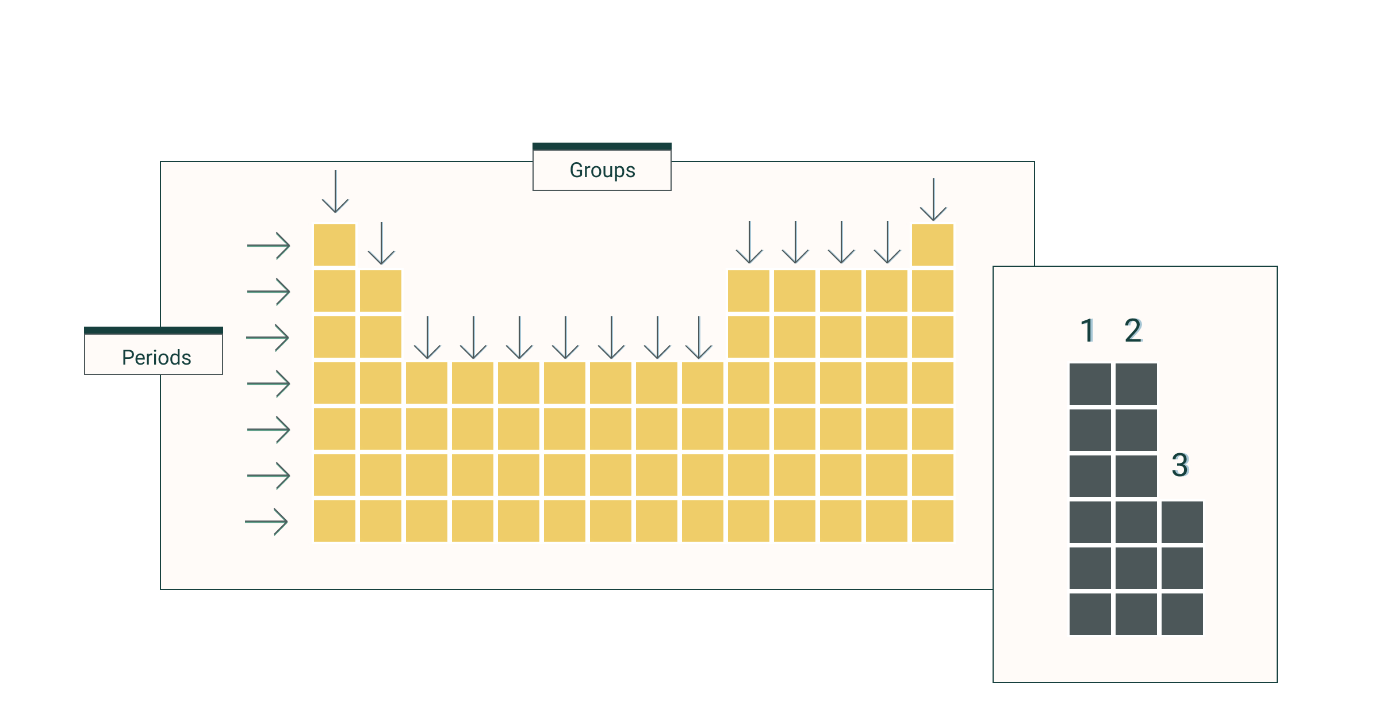
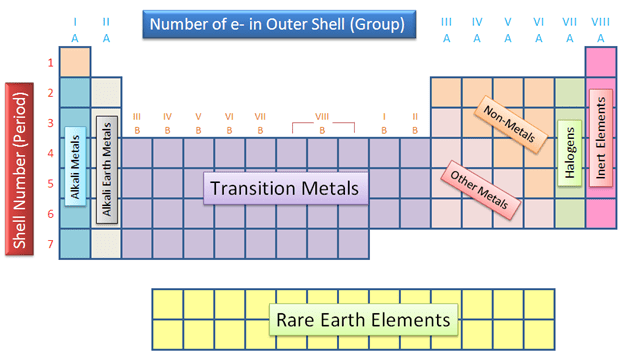
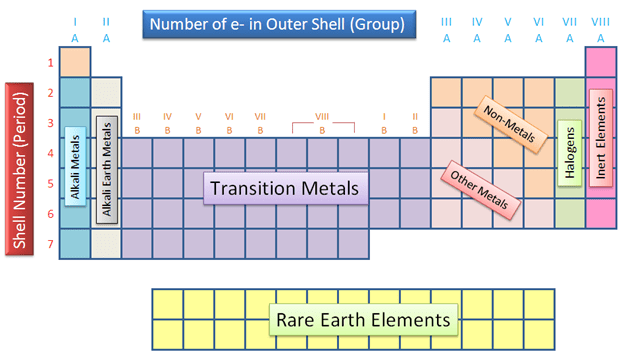
What do we call the rows on the periodic table?
Periods
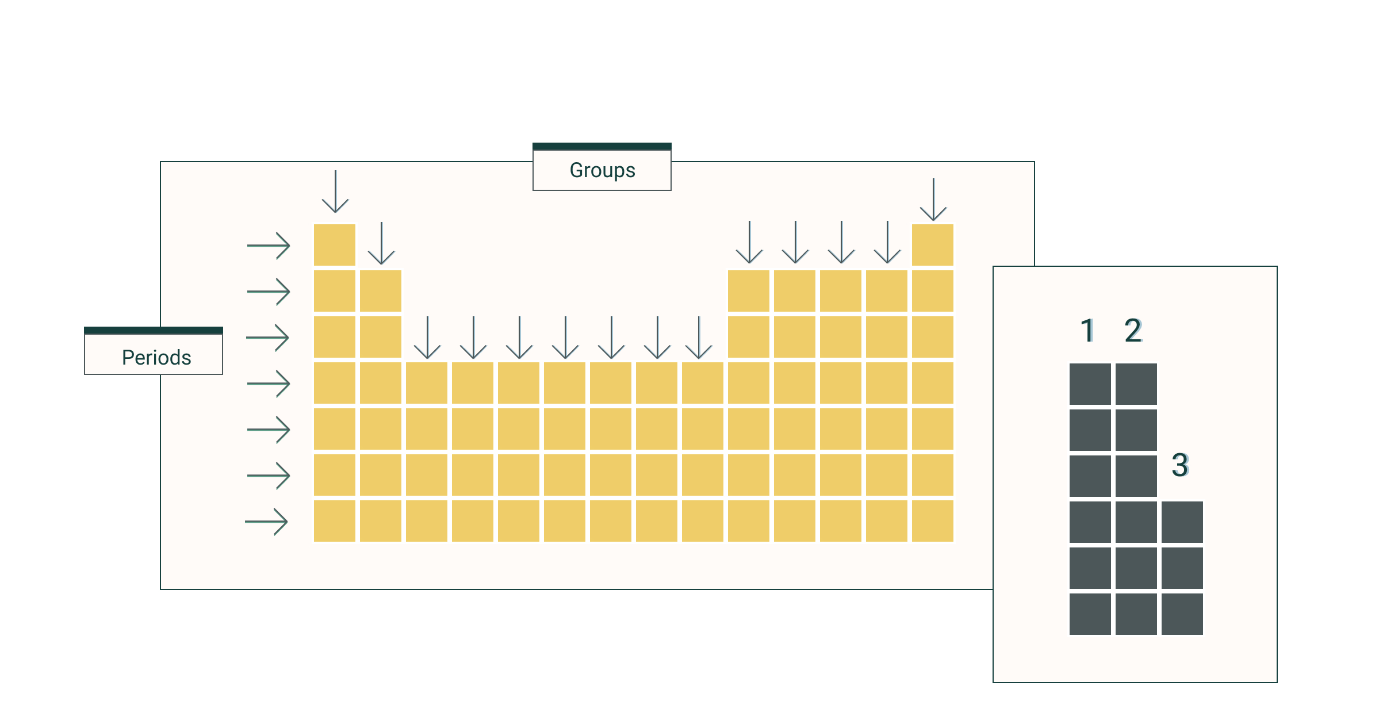
The overall charge of an atom is ___.
neutral
The smallest unit of an element that maintains the properties of that element
atom
____________ is defined as anything that has mass or volume.
Matter
What state of matter has a definite volume and takes the shape of its container?
A liquid

When melting occurs, are the particles gaining or losing energy?
When melting occurs, the particles are gaining energy. They absorb enough energy to partly overcome the force of attraction holding them together. This allows them to moveout of their fixed positions and slip over each other.

The center part of an atom that contains 2 of the 3 subatomic particles is called the atom’s __________.
nucleus
What do we call the columns on the periodic table?
Groups or Families
The electron configuration for Lithium is __
1s1, 2s1
a fixed composition
Is the following an example of a physical or chemical change? Explain why.
Ice Cube ---> Puddle of Water
Physical Change because its just a phase change
What state of matter has no definite shape and no definite volume?
A gas
What is the process in which a solid changes directly to a gas without going through the liquid state?
Sublimation
Describe each part of an atom in terms of its location.
Protons and neutrons make up the nucleus, while electrons move around the nucleus in the energy levels
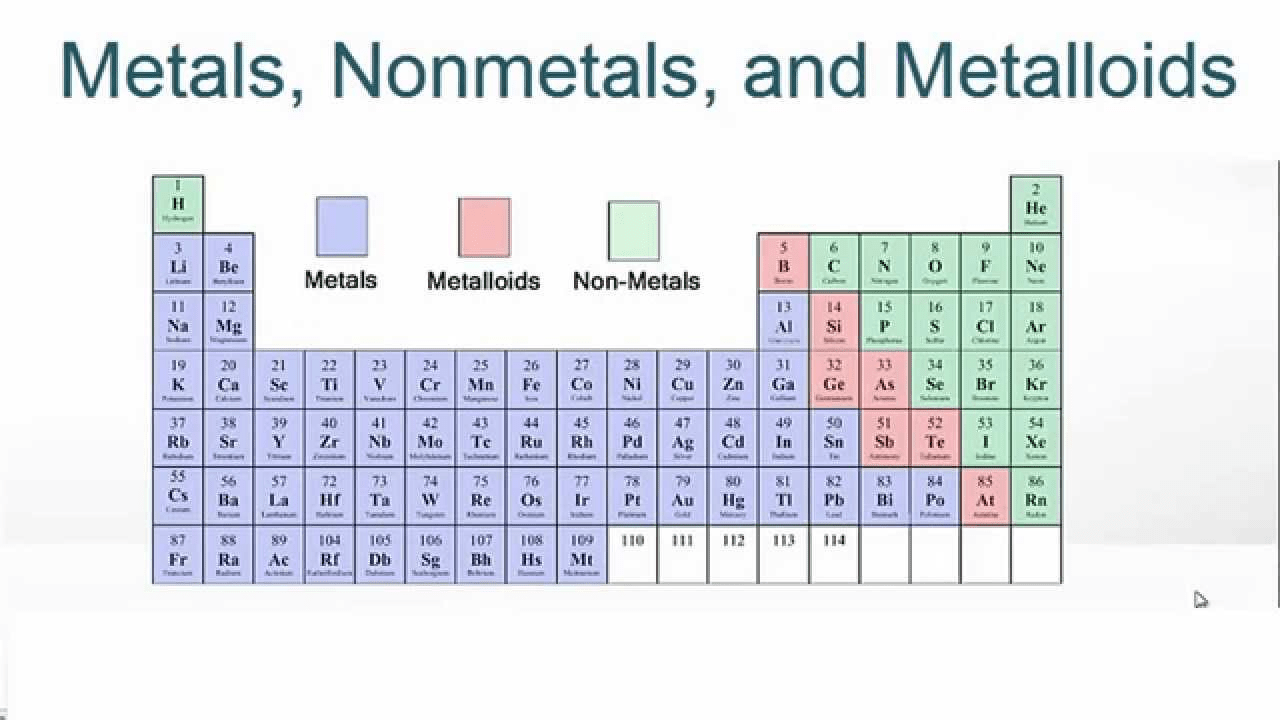
What are the three categories of elements on the periodic table?
The three major categories on the periodic table are the metals, nonmetals and metalloids.
What’s the difference between groups and periods on the periodic table?
Groups are the vertical columns while periods are the horizontal rows.
What is the study of matter and its changes called?
Chemistry
Explain the difference between exothermic and endothermic reactions.
Exothermic gives off heat and releases energy. Endothermic takes in heat and requires energy.
What state of matter will have HIGH DENSITY because there is less air between their particles?
Solid
What is the process in which a liquid changes to a gas (boiling)?
Evaporation
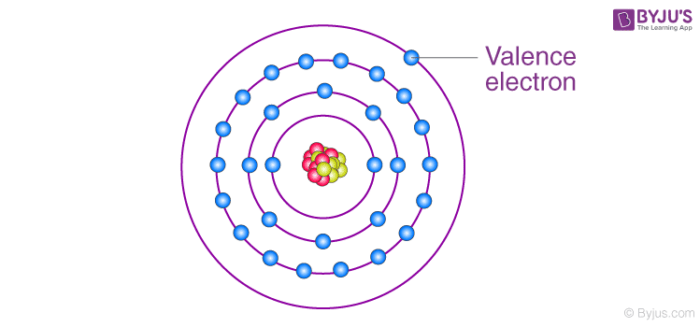
What do we call the electrons in the outermost energy shell?
Valence electrons
What are the first two groups on the periodic table called?
Alkali and alkaline earth
What is the maximum amount of a solute that will dissolve in a given quantity of solvent at a given temperature and pressure?
Solubility
An iron atom has an atomic mass of 56. Its atomic number is 26. How many neutrons does the iron atom have?
30
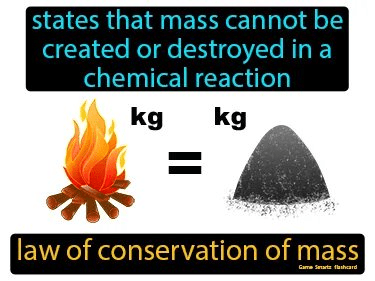
What is the law of conservation of mass?
Describe solids, liquids, and gases in terms of the distance between their particles.
From solids to gases, the particles move further apart.
At a constant temperature, as the pressure of a gas decreases, its volume __.
increases
What must happen for an electron to jump to a different energy level?
The energy level must be full
What are groups 17 & 18 on the periodic table called?
Halogens and noble gases
The order of elements in the periodic table is based on ___.
the number of protons in the nucleus
According to Bohr’s model of the atom, electrons behave like ___.
planets orbiting the sun