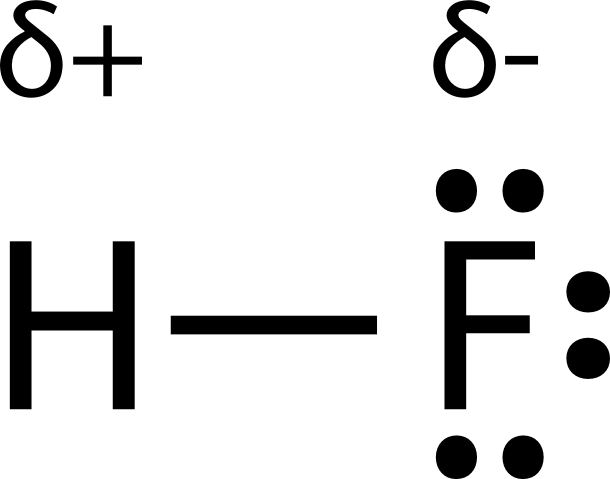According to the chart provided, what corner has the lowest electronegativity?
What is the lower left?
This happens to the atom's ability to hold electrons as the distance between the electrons and the nucleus increases.
What is becomes weaker?
Nonpolar covalent
Polar covalent
Ionic
Does HF have any dipoles? Why or why not?
Yes, HF is polar due to the electronegativity of the bond.
Does N2 have any dipoles? why or why not?
No, it is not polar
Explain why water has a tendency to create beads on surfaces.
Water is polar, thus has partial charges
These partial charges attract each other
Water beads up because the molecules are more attracted to each other than the surroundings.
A covalent bond is when ___ __________ share electrons.
What is two non-metals?
Explain what chemistry this image is portraying
The image is showing a polar bond between hydrogen and chlorine. The ice cream scoops are electrons and the size of the animals is the electronegativity value. As one animal is larger (has more electronegativity) the ice cream (electrons) spend more time on the larger animal side.
An unknown liquid is shown to dissolve in water, bead up when placed on wax paper, and has a minty sent. This liquid is polar, non-polar, or ionic.
What is polar?
NaCl: The type of bond and the estimated change in Electronegativity.
What is Ionic and 2.1?
CO2 has two equal polar bonds pulling in opposite directions, balancing them out and making the entire molecule non-polar
Draw a Lewis dot structure of HF and include the following
1. the partial charges
2. the type of bond
Polar bond (1.9)