If a substance completely dissolves in a liquid, it is ...?
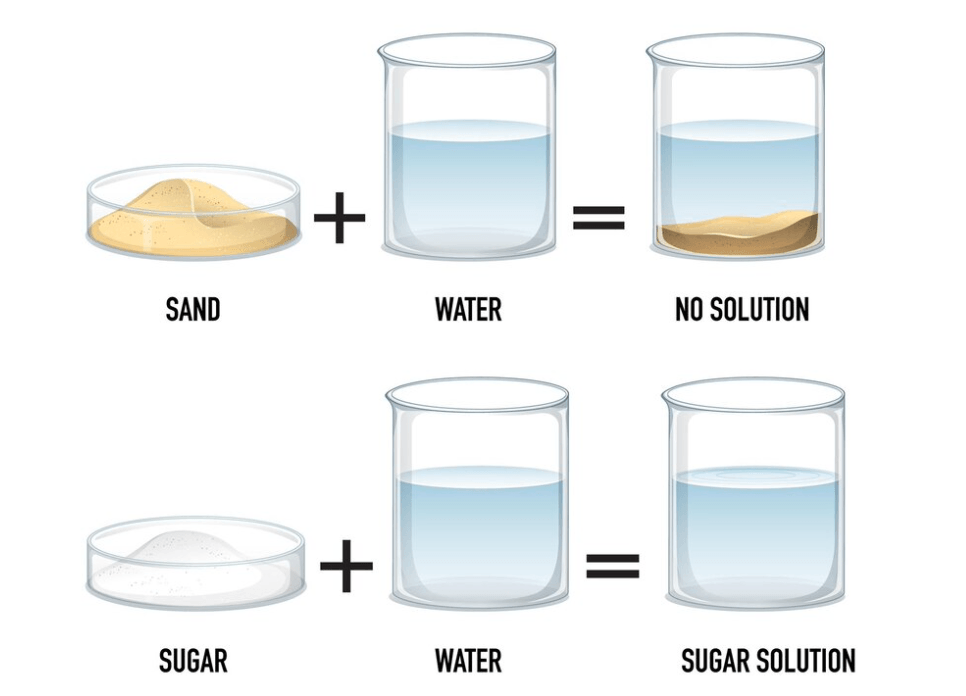
Soluble or Solubility
Rubbing Alcohol and Water look similar, but they have 1 very different chemical property, what is it?

Rubbing Alcohol is flammable.

The object floats in water, therefore the ............ is larger than the ...........
Volume larger than Mass
Which is less dense?
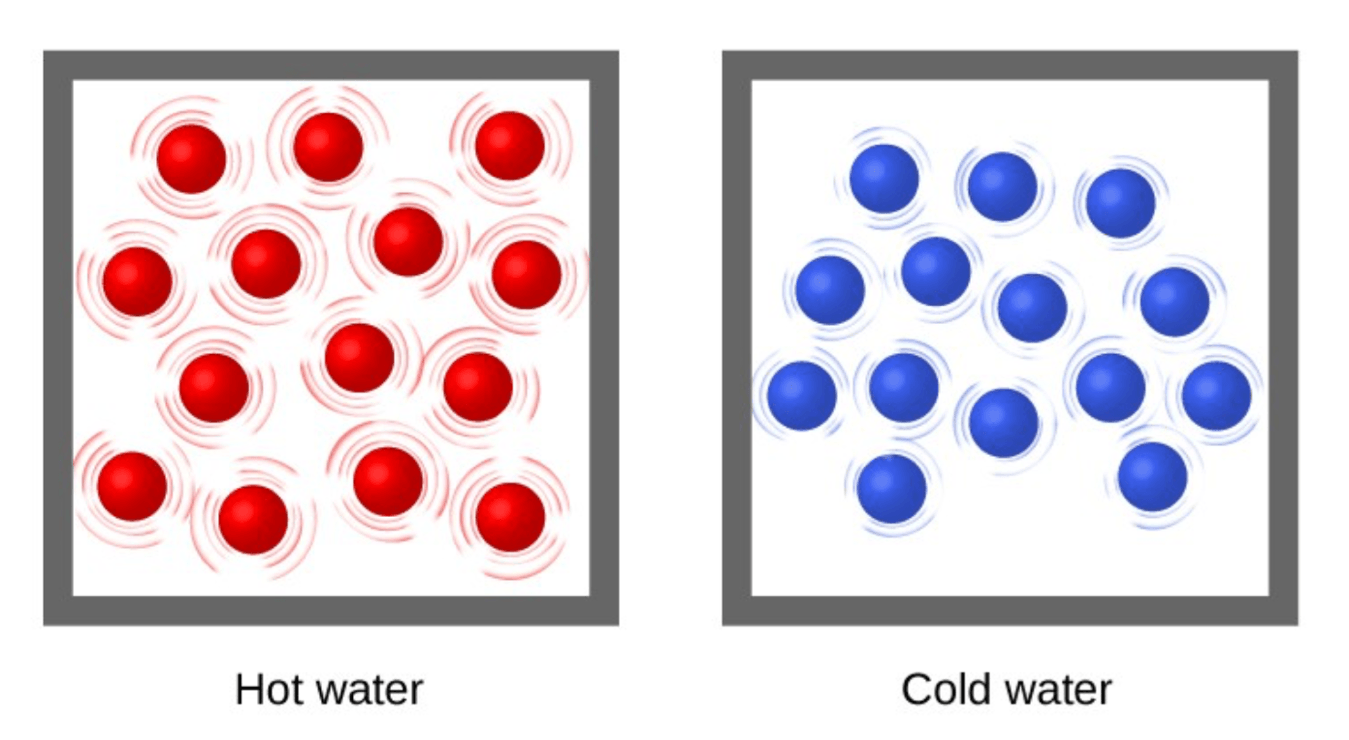
hot water
What is melting point?
Temperature at which a substance changes from solid to liquid.
Some metals, like iron react with oxygen. This process is often called ........

Rusting or Oxidization
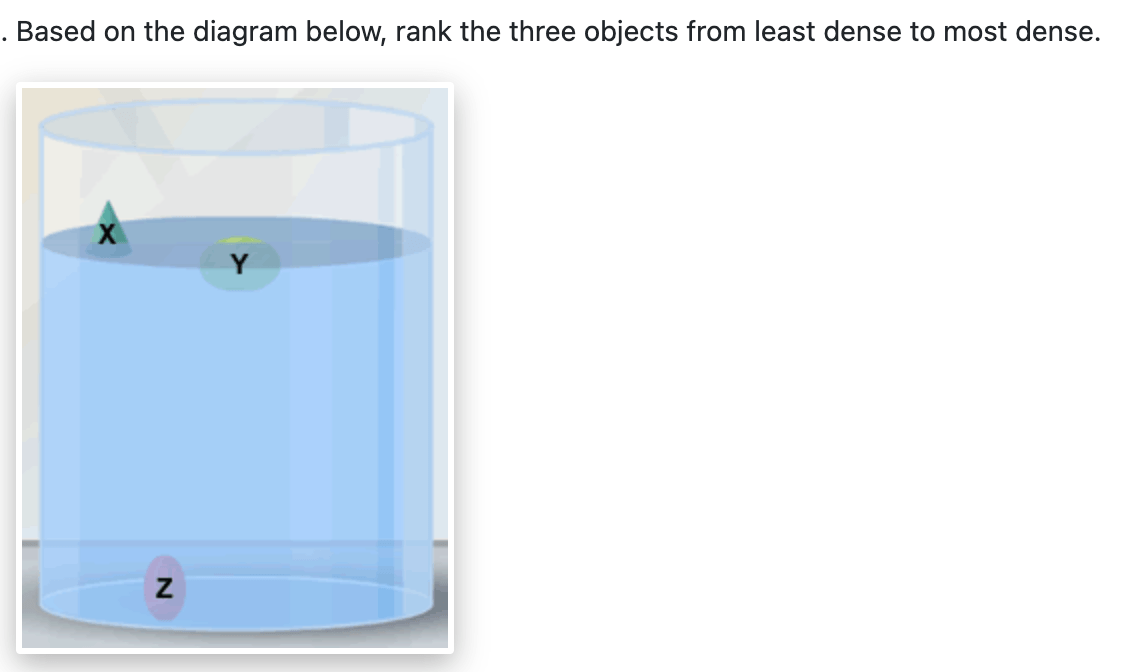
X, Y , Z
A ball has a mass of 33.6 grams and a volume of 14.0 cm3 . What is the density of the ball?
2.4 g/cm3
What is texture?

The way something feels
How are physical and chemical properties different?
Chemical properties can only be observed when there is a CHANGE. Physical properties can be observed or measured WITHOUT causing a CHANGE in substance.

B) The seawater is denser than the plain water.
Which is more dense?
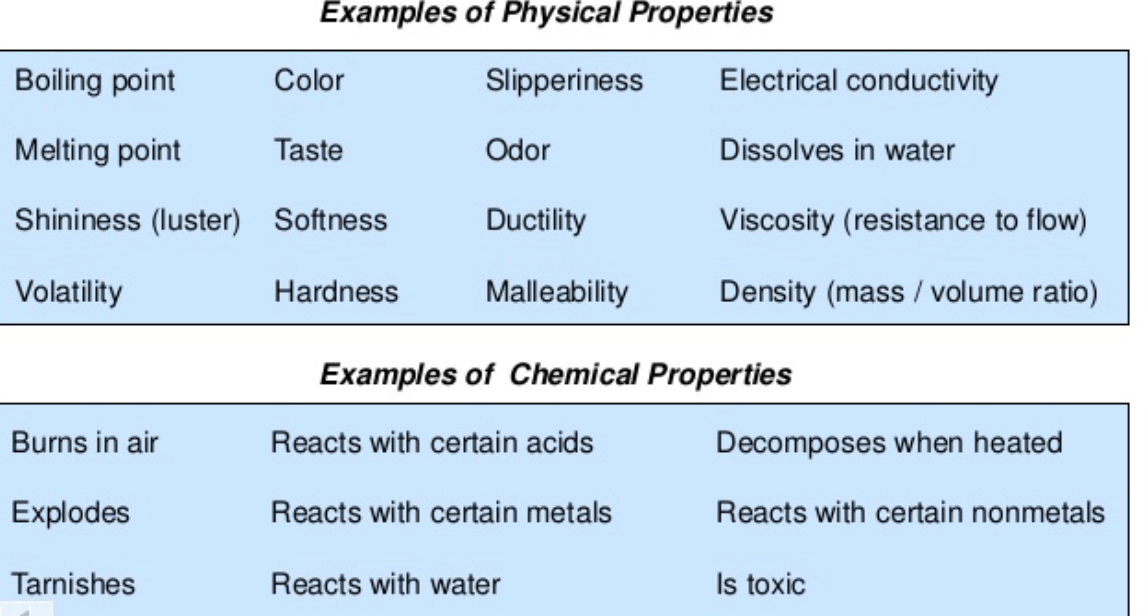
Copper
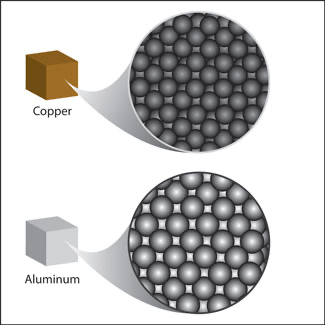
If electricity or heat is able to flow through a substance is ......
Conductive
The density of a block of Aluminum is 2.7 g/cm3 . It's measurements are a mass of 98.28 grams and a volume of 36.4 cm3.
You cut the aluminum into smaller pieces.
The smaller piece has a mass 11.91 grams and a volume of 4.4 cm3 .
What is the density of the smaller piece?
2.7 g/cm3
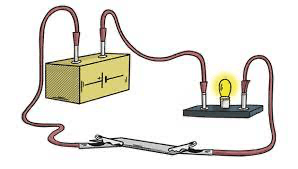
What is the density of the crown? Use the mass from the scale and the volume from the water displacement in the graduated cylinder.
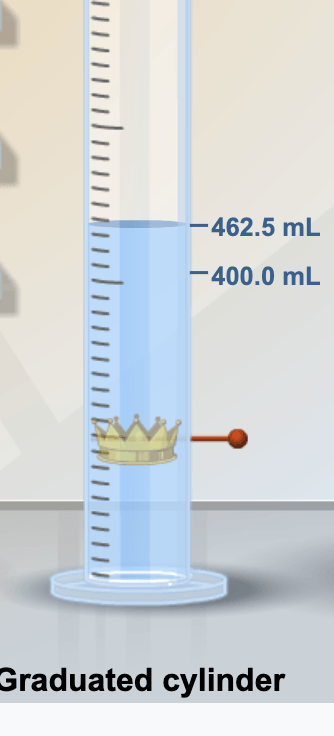
5.2 g/ml
Name 6 physical properties.
color melting point hardness
texture boiling point shininess/luster
solubility conductivity state of matter
mass volume density
magnetic odor
A deflated balloon sinks in water, but an inflated balloon floats on water. Why?

When inflating, you increased the volume. The volume became larger than the mass.
These liquids all have different densities, how would they layer from BOTTOM to TOP?
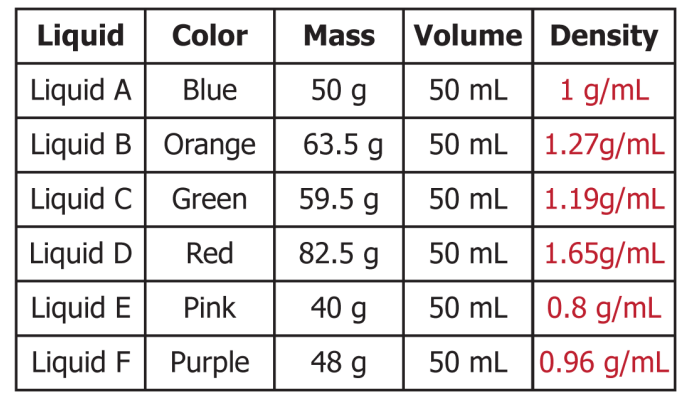
Bottom --> Top: D, B, C, A, F, E