Describe the difference between a physical and chemical property
P: characteristic that describes a substance without it changing (size, color, texture, etc.)
C: characteristic that describes a substance's ability to change (flammable, reactive, etc.)
Describe the difference between a physical and chemical change?
P: a change of a substance (size, shape, form) without changing the composition (still the same substance)
C: a change of a substance that results in a change in the composition (new substance is formed)
Describe the shape and volume of solids, liquids, and gases (definite or indefinite).
S: definite shape and volume
L: definite volume, but indefinite shape
G: indefinite shape and volume
What is needed for a change of state to occur?
Change in energy (heated or cooled)
In what part of the graph, would the substance be a solid, liquid, and gas?
Solid: I
Liquid: III
Gas: V
What is the difference between malleability and ductility? How are they similar?
M: the ability of a substance to be pressed into thin sheets
D: the ability of a substance to be pulled into a wire
Both change the shape/size of the substance (physical)
Describe 5 indicators of a chemical change.
change in color, odor
heat is absorbed or released
gas, sound, light, or precipitate is produced
Describe the arrangement and motion of the particles in a solid, liquid, and gas.
S: tightly packed, vibrate in place
L: loosely packed, can move past each other
G: far apart, move freely
What will happen when you add energy (heat) to a solid? What is this process called?
change to a liquid
melting
What is the melting point of the substance?
What is the boiling point of the substance?
MP: 5oC
BP: 55oC
What is the difference between flammability and reactivity? How are they similar?
F: the ability of a substance to burn
R: the ability of a substance to react with another substance
Both are chemical properties
All phase changes are what kind of change? Why?
Physical
doesn't form a new substance, just changes from solid to liquid to gas
Describe the relationship between volume, temperature, and pressure of a gas.
When one is held constant, how does changing one affect the other?
Boyles: T constant, As P increases, V decreases (inverse=opposite)
Charles: P constant, As T increases, V increases (direct=same)
G-L: V constant, As T increases, P increases (direct=same)
What will happen when you remove energy (cool) from a gas? What is this process called?
change into a liquid
condensation
For how long was the substance in the liquid phase?
10 min
What is the difference between solubility and viscosity? How are they similar?
S: the ability of a substance to dissolve
V: a substance's resistance to flow
Both are physical properties, involve liquids
A pumpkin is cut open and the seeds are removed. A face is carved into the pumpkin. The pumpkin is set outside and after a few days, the pumpkin begins to rot.
Identify the different changes as physical or chemical and explain why.
Cutting: P
Carving: P
Rotting: C

B
Describe the kinetic energy of a substance as it changes state from solid to liquid to gas.
particles gain energy, speed up and move farther apart
In what part of the graph would the substance have the greatest kinetic energy?
In what part of the graph would the substance have the least kinetic energy?
Most: V (gas)
Least: I (solid)
What is the difference between the melting point and freezing point of a substance? How are they similar?
MP: change from solid to liquid
FP: change from liquid to solid
Both happen at the same temperature (water freezes at 0 degrees C and ice starts to melt at 0 degrees C)
On your camping trip, you want to make s'mores. First you chop wood for the campfire. Then you start the fire and the wood begins to burn. You put the marshmallow on the stick and roast it over the fire, but it got too close and caught on fire and charred the outside. You then break apart the graham cracker and chocolate bar to finish assembling your s'more.
Identify the different changes as physical or chemical and explain your reasoning.
Chopping: P
Burning: C
Roasting/Charred: C
Breaking Cracker and Chocolate: P
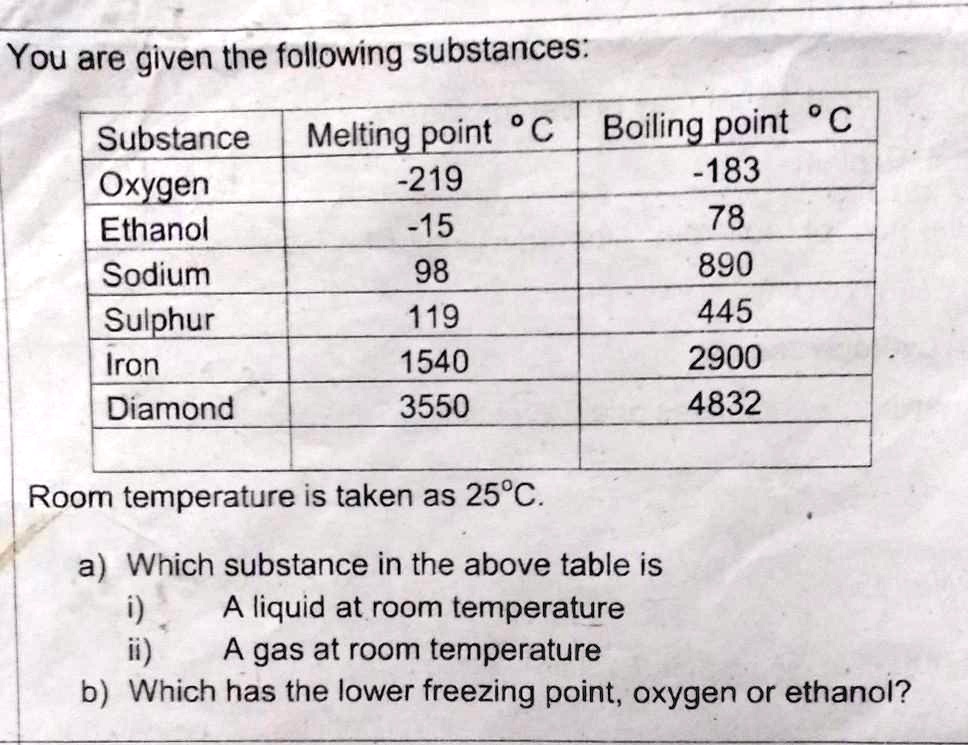
a) i) ethanol (25 between -15 and 78)
ii) oxygen (25 is above -183)
b) oxygen (FP and MP are the same)
Describe the kinetic energy of a substance as it changes state from gas to liquid to solid.
particles lose energy, slow down and move closer together
Describe the temperature during the two phase changes.
II (melting) and IV (boiling)
the temperature is constant (doesn't change) during a phase change