Which statement defines matter?
A Matter is anything that has weight and height.
B Matter is anything that has weight and mass.
C Matter is anything that has mass and volume.
D Matter is anything that has height and volume.
What is matter is anything that has mass and volume?
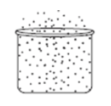
Look at the diagram. Which phase of matter does the diagram most likely represent?
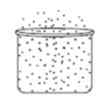
Gas
Liquid
Mass
Solid
What is a gas?
Which term describes a substance that was formed by the combination of two or more elements?
Atom
Compound
Element
Molecule
What is a compound?
Which part of the atom has a negative charge?
Electron
Proton
Neutron
Nucleus
What is an electron?
The diagram shows particles of water in three different states of matter labeled E, F, and G.
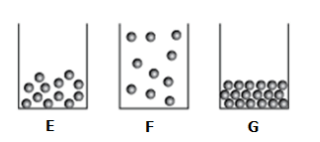
Which states best classify E, F, and G?
Possible Answers: ice, water, water vapor
What is E is water, F is water vapor, and G is ice?
Mr. Williams told his fifth grade students they could
take the sports equipment shown out for recess.

Which statement is true about all of the pieces of sports equipment?
A All of the pieces of sports equipment are the same size.
B All of the pieces of sports equipment are made of matter.
C All of the pieces of sport equipment are the same shape.
D. All of the pieces of sports equipment have the same volume.
What is all of the pieces of sports equipment are made of matter?
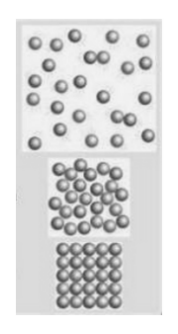
What is the correct order of the states of matter from top to bottom?
Solid, liquid, gas
Gas, solid, liquid
Gas, liquid, solid
Solid, gas, liquid
What is gas, liquid, solid?
When two oxygen atoms chemically combine with 2 hydrogen atoms, hydrogen peroxide (H₂O₂) is formed. Which term describes hydrogen peroxide?
Element
Compound
Atom
Mixture
What is a compound?
Emily conducts an experiment to compare the densities of cork and iron with water. She places a piece of cork and a small iron nail in a beaker filled with 100 mL of water, and she records her observations.

What can Emily conclude from her experiment?
Both the cork and iron nail are more dense than water.
Both the cork and iron nail are less dense than water.
The cork is more dense than water, and the iron nail is less dense than water.
The iron nail is more dense than water, and the cork is less dense than water.
What is the iron nail is more dense than water, and the cork is less dense than water?
Lillian’s balloons are filled with a type of gas called helium. How do the particles inside Lillian’s balloon move?
The particles are very close together and are vibrating.
The particles remain in place and do not move.
The particles are close to one another, but are able to move away from each other and flow from pace to place.
The particles easily move far apart from each other and spread out to fill the available space.
What is the particles easily move far apart from each other and spread out to fill available?
A teacher uses blocks to make three models (P, Q, and R). The teacher asks a student to pick one model to represent an atom.
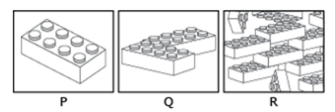
The student picks model P. How should the student correctly explain this choice?
A. Model P occupies the largest area.
B. Model P cannot be broken down further and still have the properties of the block.
C. Model P is the unit used to build models Q and R but has different properties.
D. Model P can be broken down further.
What is model P cannot be broken down further and still have the properties of the block?
Trisha was at the circus and noticed people walking around with these three differently shaped balloons filled with helium gas.
Which is the best conclusion Trisha could make about the gases after she observed these balloons?
Gases have indefinite shapes and volumes.
Gases have definite sizes and shapes.
Gases have definite shapes and volumes.
Gases’ sizes and shapes do not change.
What is gases have indefinite shapes and volumes?
Select all of the substances that are compounds.
Ozone (O₃)
Water (H₂O)
Carbon Dioxide (CO₂)
Sodium Chloride (NaCl)
Iron (Fe)
What is water, carbon dioxide, and sodium chloride?
Peter wants to know the volume of object A. He fills a graduated cylinder with 10mL of water, and then places object A inside the graduated cylinder. He draws a before and after diagram in the science notebook.
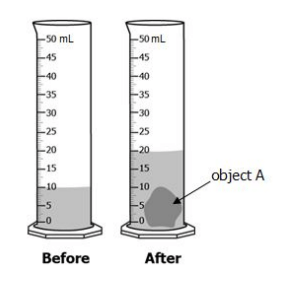
What is the volume of object A?
20 mL
15 mL
10 mL
5 mL
What is 10 mL?
Examine the figures.
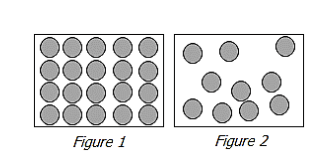
Which figure represent a solid, and what evidence supports it?
Figure 1 because the particles are tightly packed.
Figure 2 because the particles are tightly packed.
Figure 1 because the particles are far apart.
Figure 2 because the particles are far apart.
What is figure 1 because the particles are tightly packed?
Which statement best describes a molecule?
A molecule is always a group of the same atoms bonded together.
A molecule is always two or more substances in a mixture.
A molecule is always two or more atoms bonded together.
A molecule is always a solution.
What is molecule is always two or more atoms bonded together?
A scientist uses an instrument to study the small particles in a piece of matter. The diagram shows what the scientist sees.

What state of matter is the scientist most likely observing?
Gas
Liquid
Solid
Vapor
What is a solid?
All matter is made of atoms. Which drawing shows the correct relationships between the three basic parts of an atom?

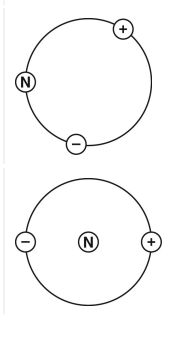
What is choice B?
Allie observes Substance X and Substance Y. She lists some differences between the two substances in a table.
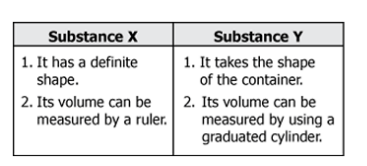
Which substances most accurately match Allie’s description?
Substance X is oil, and substance Y is water.
Substance X is a wood cube, and substance Y is water.
Substance X is water, and substance Y is oil.
Substance X is water, and substance Y is a wood cube.
What is substance X is a wood cube, and substance Y is water?
When an electrician makes repairs to the wires that carry electrical current throughout an entire house, he must use a special plastic tape. Which statement would best explain why the electrician must use the special plastic tape?
The plastic tape will help keep the wires from overheating.
The plastic tape will help make the wires more flexible.
The plastic tape will act as insulator of the electrical current.
The plastic tape will last for a very long time.
What is the plastic tape will act as insulator of the electrical current?
The diagram shows atoms and molecules.
What is true about the diagram?
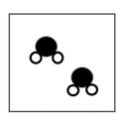
The atoms are made up of three molecules.
Each molecule is made up of two atoms.
The atoms are made up of two molecules.
Each molecule is made up of three atoms.
What is each molecule is made up of three atoms?
Which term describes the smallest part into which an element can be divided and still maintain its properties?
Atom
Compound
Element
molecule
What is an atom?
Which of these is true about parts that make up atoms?
Protons are larger than neutrons.
Electrons have a positive charge.
Electrons are inside the nucleus.
Neutrons have no charge.
What are neutrons have no charge?
Sarah packs grape juice for her lunch one day. Which statement best describes the grape juice?
The grape juice has a definite shape and volume.
The grape juice has a definite volume, but no definite shape.
The grape juice has no definite shape or volume.
The grape juice has a definite shape and no volume.
What is the grape juice has a definite volume, but no definite shape?
A student makes a partial circuit by connecting a battery with a light bulb using wires. The student then completes the circuit using different materials to check if the light bulb glows, as shown in the setup and table.
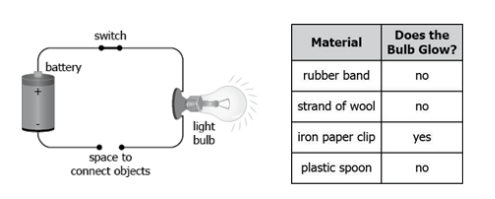
Which material can be classified as an electrical conductor?
Iron paper clip
Plastic spoon
Rubber band
Strand of wool
What is iron paper clips?