Anything that has mass and takes up space
matter
Gas particles move ____ and _____
fast and separated
True or False: solid particles are packed tightly together
True
What is the Density of Water
1 g/ml
What is this color of a mineral in powder form called?
streak
The four states of matter are...
solid, liquid, gas, plasma
Gases ______ into a liquid. Liquids _______ into a gas.
condense, evaporate
I have a small sample of a gray metal. What would help me identify the metal accurately?
density
If a property can be observed without changing the identity of the substance, it must be a
physical property
Objects with a density less than water will ______
float in water
Density refers to a physical property that is measured using a ratio of mass divided by ______
volume
Methane is a colorless, odorless gas. It oxidizes and has a boiling point of -161 C. what is the chemical property
oxidation
Less dense objects are more _______
buoyant
Chemical properties of matter depend mostly on
types of atoms and bonds that a substance contains
true or false ; Density will always stay the same
true
68.0g of mercury occupies a volume of 5.00cm3. Calculate the density of mercury in g/cm3.
13.6 g/cm3
Most substances _______ in temperature and _______ when heat is added to them.
increase; expand
_______ is a _______ property of a substance and is detected by the sense of smell
Odor; physical
You have a rock with a volume of 15 cm3 and a mass of 45 g. What is its density?
3.0 g/cm3
A wooden beam is 3.0m long and has a volume of 0.12m3. Its mass is 60kg. What is its density?
500 kg/m3
List the buzz words of chemical properties
react
rust
burn
oxidize
Fill in the blanks for
1
2
3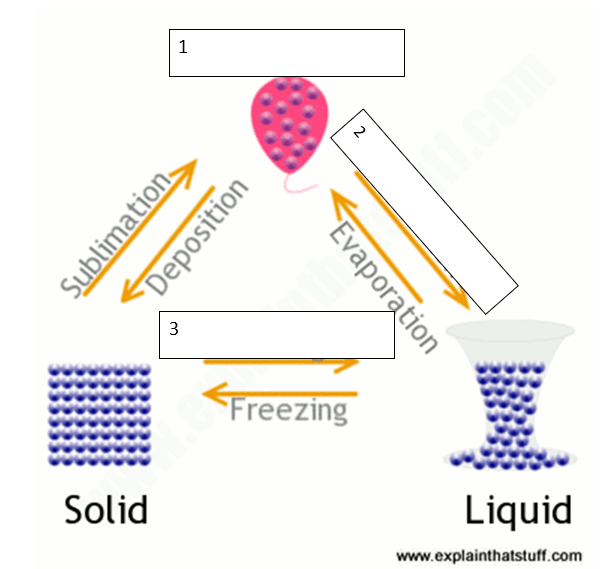
1. Gas
2. Condensation
3. Melting
List 6 of the physical properties of matter
Texture
Color (streak test)
Shape
Mass/ Weight
Volume (space it occupies)
Odor
Solubility
Magnetism
Conductivity
Melting pt (solid to liquid)
Freezing pt (liquid to solid)
Boiling pt (liquid to gas)
Thermal expansion
Thermal contraction
Density
Buoyancy
Fill in the blanks
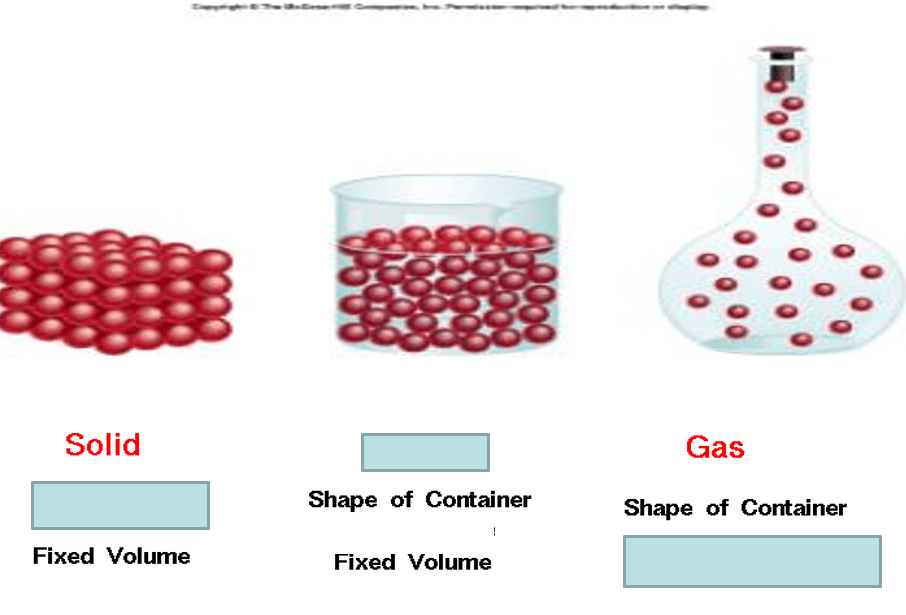
holds shape
Liquid
Volume of container
What is this called?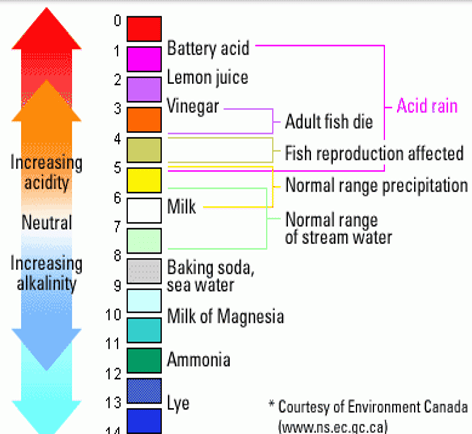
pH Scale