The amount of matter inside an object
What is Mass?
This happens to the molecules in an object as energy is added to them.
They have more motion/move more.
Elements and compounds make up this type of matter
What is (pure) substance?
This formula is used to find the density of a object.
What is D=m/v?
THe point at which a liquid will become a gas?
What is boiling point?
This is a picture of what state of matter.
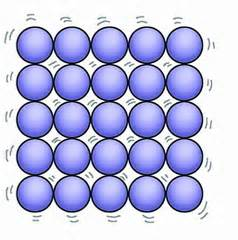
What is a Solid?
I am not alive, but I grow; I don't have lungs, but I need air; I don't have a mouth, but water kills me. What am I?
a fire
The amount of space an object takes up.
What is Volume?
If energy is added to a solid substance, what happens to the substance?
It liquefies/melts
Another word for a homogeneous mixture.
What is solution?
A pure substance made of only one type of atom.
What is an element?
The boiling point of water, in Celsius.
What is 100 degrees C?
This is a picture of a shocking state of matter.
/lightning-58b5e8e95f9b5860460c3eab.jpg)
What is Plasma?
 Why can't the Tyrannosaurus Rex clap?
Why can't the Tyrannosaurus Rex clap?
Because they are extinct.
The upward force of a fluid on an object.
What is Buoyancy?
What is the theory called that states that all matter is made of particles in constant random motion?
Kinetic molecular theory
The characteristic of matter that may be observed and measured without changing the chemical identity of a sample..
What is physical property?
The scattering of a beam of light by a medium containing small suspended particles—e.g., smoke or dust in a room, which makes visible a light beam entering a window..
What is the Tyndall Effect
Find the density of an object with a mass of 280g and a volume of 4cm3.
What is 70g/cm3?
This is the name of the process that causes a solid to become a gas.
What is sublimation?
If Fortune had a daughter, what would her name be?
Misfortune
the liquids resistance to flow.
What is viscosity?
This happens to the molecules of a substance if energy is removed from them.
They move less/slow down.
This type of mixture has uniform composition throughout
What is homogeneous mixture?
A heterogeneous mixture in which very small particles of one substance are distributed evenly throughout another substance. The particles are generally larger than those in a solution, and smaller than those in a suspension.
What is a colloid?
The freezing point of water.
What 0 degrees C?
This is a picture of this "fluid" state of matter.
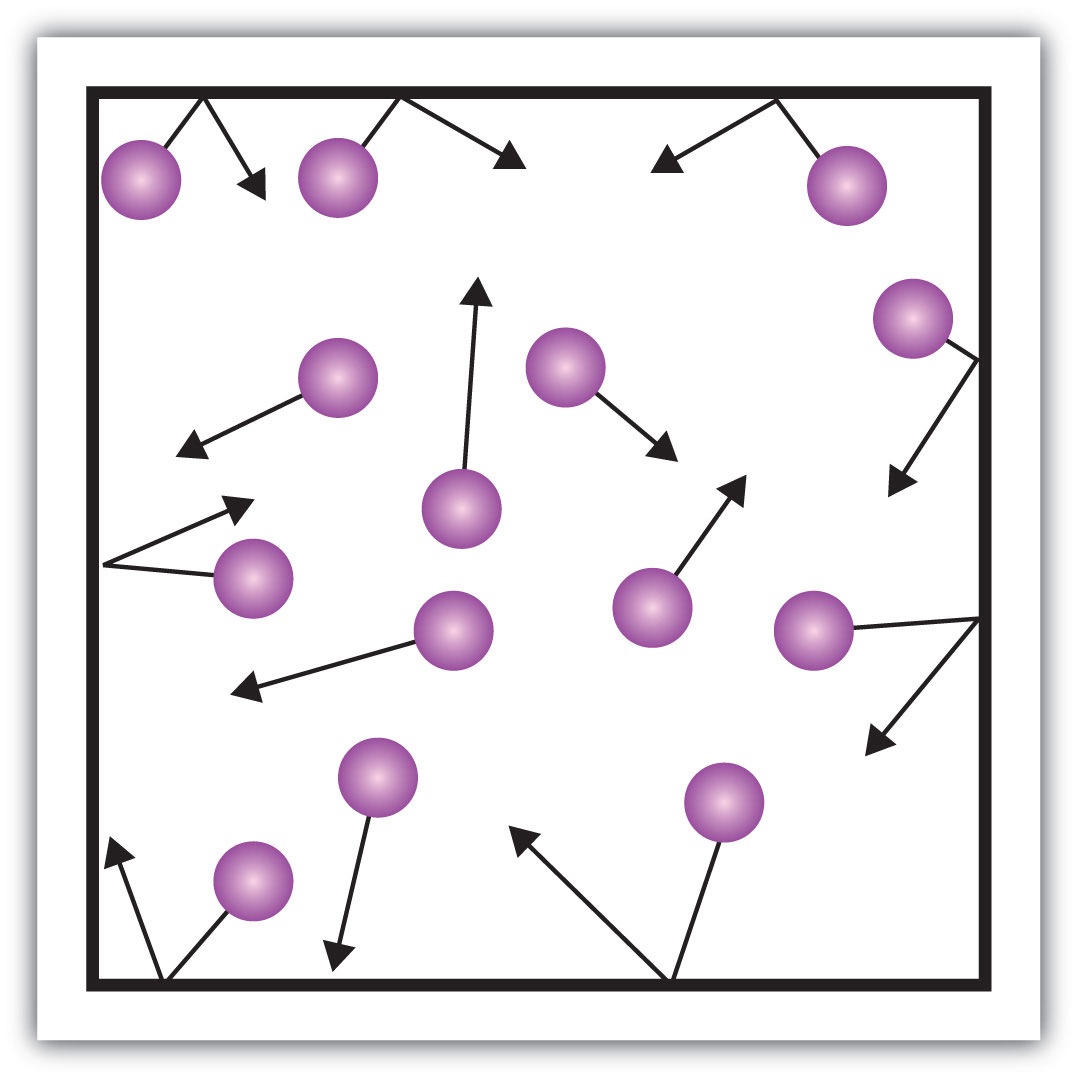
What is a gas?
 Why are all Superman costumes tight?
Why are all Superman costumes tight?
Because they're all size S.
The amount of mass in a given volume.
What is Density?
What phase of matter, common on Earth, that has the most molecular motion?
Gas
Any substance consisting of two or more different types of atoms (chemical elements) in a fixed stoichiometric proportion or ratio.
What is a Compound?
characteristic of a substance that may be observed when it participates in a chemical reaction
What is chemical property
physical law of buoyancy, discovered by an ancient Greek mathematician and inventor, stating that any body completely or partially submerged in a fluid (gas or liquid) at rest is acted upon by an upward, or buoyant, force, the magnitude of which is equal to the weight of the displaced fluid
What is Archimedes Principle.
This is a picture of another fluid state of matter.
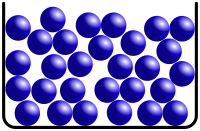
What is a liquid?
There's a bathtub filled with water in front of you. You are given a cup, a spoon, and a bucket. What do you use to get the water out of the bathtub?
The drain.