The state of matter where molecules are spread very far apart.
What is gas?
Also accepted: What is plasma?
Made of identical atoms in the same combination.
What is a pure substance?
The types of properties that can be observed without changing the identity of the substance.
What are physical properties?
The process matter undergoes when it turns from a liquid into a solid.
What is freezing?
The smell of a cake cooking is evidence of this type of change in matter.
What is chemical change?
The state of matter where molecules are close but can slide past each other.
What is liquid?
Contains two or more substances that are not chemically combined.
What is a mixture?
This formula represents this property of matter.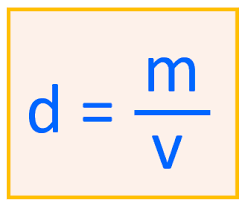
What is density?
The process matter undergoes when it turns from a liquid to a gas.
What is evaportaion?
OR
What is vaporization?
When you cut an element in half to reduce it's lengeth you are changeing this property.
What is a physical property?

What is Solid.
Copper wire, aluminum foil, and gold are are examples of this type of pure substance.
What is an element?
This property of matter describes the ability of a substance to undergo changes in identity.
What is chemical property?
The process matter undergoes when it turns from a gas to a liquid.
What is condensation?
What is a precipitate?
The state where particles move quickly, spread out and take up all avaialable space, and it can cange both space and volume.
What is gas.
What is a compound?
This chemical property describes how easily something ignites and the intesity it burns.
What is flammability?
The process matter undergoes when it turns from a gas to a solid.
What is deposition?
A chemical reaction that causes heat to be produced.
What is an exothermic reaction?
This state of matter is made when particles collide with enough energy to break into charged particles.
What is plasma?
Sometimes called a solution, this type of mixture contains very small particles where the particles are dissoled in a liquid, but not chemically combined.
What is a homogeneous mixture?
This chemical property describes how much a substance can damage a cell, organ or organism.
The process matter undergoes when it turns from a solid to a gas.
What is sublimation?
When energy is absorbed in a chemical reaction.
What is an endothermic reaction?
This is the amount of atoms an object contains.
What is mass?
A mixture where particles don't dissolve and may or may not settle out.
What is a heterogeneous mixture?
The ability to react with oxygen causing the loss of electrons.
What is oxidation?
To cause a solid to melt into a liquid you would have to do this.
What is increase heat energy?
When observing a chemical reaction, the producation of bubbles is evidence of this chemical change.
What is the formation of a gas?