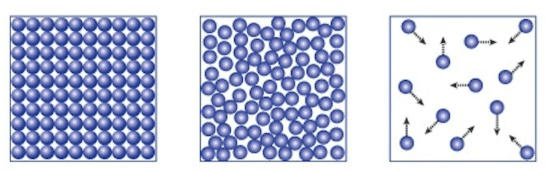
 Which state of matter is each illustration representing?
Which state of matter is each illustration representing?
1. Solid
2. Liquid
3. Gas
Is a salad homogeneous or heterogeneous?
Heterogeneous
Is a piece of wood floating on water more dense OR less dense than water?
The piece of wood is less dense than water because it is floating on water.
A state of matter that has no fixed shape and no fixed volume
Gas
What is the definition of Homogeneous Mixture?
A mixture that looks the same throughout (uniform).
Which of these liquids will sink below the others?
Mercury
A _______ is a state of matter with a fixed shape and volume.
Solid
What is the definition of Heterogeneous Mixture?
A mixture that does not look the same throughout ( not uniform).

Which of these liquids will float on top of the others?
Olive Oil
A state of matter whose shaped changes with its container, but their volume stays the same
Liquid
The definition of a Pure Substance
Substances that are made up of only one kind of substance.
A substance that cannot be broken down physically!
Examples are elements and compounds!
Like Oxygen and Salt
A measure of the amount of mass present in a given volume of a substance.
Density
Explain how the atoms in a solid, liquid, and gas MOVE.
Solids vibrate.
Liquids move past one another.
Gases bounce around quickly.
Give me an example of a Pure Substance, a Heterogeneous mixture, and a Homogeneous mixture.
STUDENT EXAMPLE
Density describes the relationship between ____ and ____.
mass and volume
A balloon filled with air drops to the ground slower than a balloon filled with water.
Using what you have learned during this unit, explain the scientific reasoning for why this happens.
A balloon filled with water is more dense than a balloon filled with air.
Explain the difference between a homogeneous mixture, heterogeneous mixture, and a pure substance.
A homogeneous mixture becomes uniform and looks the same throughout.
The substances that makes a heterogeneous mixture keep their properties.
A pure substance is only one kind of substance that cannot be broken down.
 If I had a tower of liquids and I wanted to figure out which one was water... What could I do to determine which layer was the correct one.
If I had a tower of liquids and I wanted to figure out which one was water... What could I do to determine which layer was the correct one.
Pour water into the tower and watch which layer changes.