Blue color is an example of
What is a physical property?
An apple is left out and rots. What 2 indicators would tell us that a chemical change occurred?
-Change in color
-Smell
Carbon dioxide is an example of?
What is a compound?
Provide one example of a mixture.
Answers will vary.
Boiling point, melting point and density are some of an element’s
Physical properties
What is a physical change?
The following picture shows an example of what? 
What is an molecule?
How many different substances are in this mixture? 
What is 2?
Describe 2 types of changes that occurred during the smores lab.
Chemical: burning marshmallow, digestion
Physical: melting chocolate, breaking graham cracker, chewing
Acidity and flammability are examples of?
What is chemical properties?
An ice cube is placed in the sun. Later, there is a puddle of water. Later still the puddle is gone. What physical changes are occurring?
What are melting and evaporation? (State changes)
An element is a pure substance in which there are how many different kinds of atoms?
What is one?
Why is air considered a mixture?
Air is made up of several different compounds and molecules such as CO2, H2O, N2, and O2.
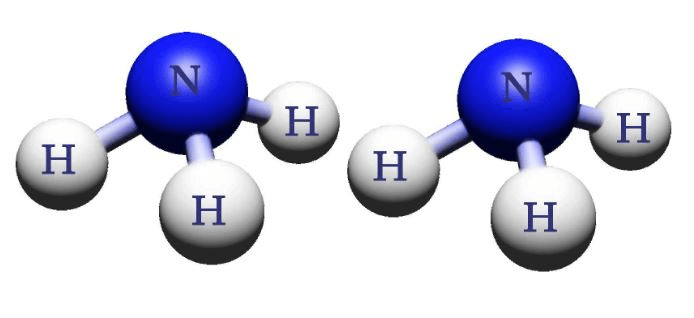
Draw the following substance:
2NH3
A student is performing an experiment with baby powder. The student notes that the powder is soft, white, and smells good. In this example, smell is a...
Physical property
We know when a chemical change has occurred when we answer yes to this important question.
Has a new substance been formed?
This is what is formed when two or more elements are joined together chemically.
What is a compound?
Salt and water are considered this.
Salt water is this.
Salt and water: compounds
Salt water: mixture
Describe the type o substances you see in the picture. Explain your answer. 
CO2 is a compound. It is made of two different types of atoms.
O2 is a molecule. It is made of the same type of atom.
Sal is given a sample of an unknown liquid to test in the laboratory. Sal thinks the liquid might be water. Which physical properties would be helpful for Sal to determine the identity of the liquid?
Acceptable answers:
1. Boiling point
2. Melting point
3. Density
A new substance is formed during a chemical change. What remains the SAME between the original and new substance?
The original and new substance should have the same mass/matter/number of molecules. The amount of matter has not changed, it was just rearranged to form the new substance.
What compound is the following?
CCCCCCHHHHHHHHHHHHOOOOOO
What is C6H12O6?
Based on your mixtures lab, how do mixtures differ from compounds? Make a claim and support with evidence from the lab.
Answer the question using evidence from the formula...is matter being created? 
No matter is not created! The same amount of matter on one side of the equation is on the other. Ex: There are 4 total hydrogen atoms on the left (H4) and 4 on the right (2H2)