The property(ies) responsible for surface tension

Cohesion.
Water's ability to stick to itself allows it to create a smooth and somewhat durable surface (at least for small insects and paper clips).
This is the variable that is manipulated in an experiment and is often plotted on the x-axis.
Independent Variable
will NaCl will dissolve in water?
It WILL!
NaCl (table salt) will dissolve in water because it is an ionic compound.
Supported or Rejected
This property explains how the sand at the beach can feel hot, while the water at the same beach remains cool
High specific heat capacity
This is the term for a molecule having a positive part and a negative part.
Polarity (or polar)
The property(ies) demonstrated by wet hair.
Adhesion as the water stuck to the hair.
In the grass experiment, the height of the plants?
Dependent variable
DV is measured
Will butter mix with water?
It Won't!
Butter is a fat which is nonpolar, which will not mix with polar water.
This is the result of countless descriptions of a natural phenomena.
Scientific law
This property allows aquatic life to survive the winter.
Lower density of ice (solid water floats)
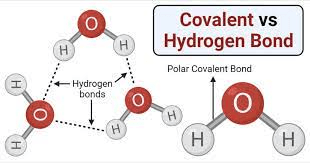
Name that bond:
The shared electrons between a hydrogen atom and oxygen atom in a water molecule
Covalent bond
The property(ies) responsible for capillary action

Both.
Adhesion- as the water sticks to the sides of the tube
Cohesion- each water molecule sticks to itself and pulls molecules below up the tube.
Shonda asks teachers to drink different amounts of caffeinated drinks and measures their typing speed.
Number of caffeinated drinks is which variable?
Independent Variable
Will polar molecules dissolve in water?
They Will!
Water can dissolve other polar molecules.
This group receives the treatment
Experimental Group
The property that keeps plants hydrated.
Capillary action due to adhesive and cohesive properties.
Name that bond:
The electrostatic attraction between two separate water molecules.
Hydrogen bond
What property(ies) are exhibited here.

Cohesion
Water sticking to itself allows for water to "pile" up and create a bead.
The variable not added to the control group
Independent Variable
Will pure water have an even number of hydrogen and oxygen atoms?
It won't!
Water is H2O - two hydrogens and one oxygen
This is the process by which scientific papers are evaluated by members of the scientific community before being published.
Peer review
The property that helps cool the body.
High specific heat.
Sweat on the skin can absorb a lot of heat before it evaporates altogether. This means that more heat can be pulled away from the body to help keep it at a constant temperature (i.e. homeostasis).
A logical interpretation based on an observation
Inference
These bonds that form between water molecules allow for their cohesive properties.
Hydrogen bonds.
Though fairly weak, the bonds formed between the slightly positive hydrogens and the slightly negative oxygens are what allow water molecules to "stick" to each other.
Plotted on the y axis
Dependent Variable
Will Ethane (pictured below) dissolve in water?
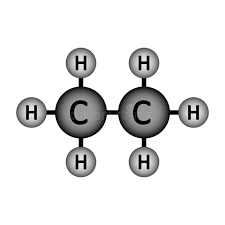
Won't!
Ethane is a non-polar molecule (because it is symmetrical). Non-polar molecules do not dissolve in water.
These variables are kept the same between the experimental groups.
Controlled variables
This property of water is ultimately responsible for cohesion, expansion upon freezing, and high specific heat.
Hydrogen bonding
The term for a single description or measurement
Observation