This number, 3.293, has this many significant figures.
4
Convert 5.2 cm to mm.
52 mm
A cube has a mass of 50 g and a volume of 10 cm³. What is its density?
5.0 g/cm³
What is the SI unit for temperature?
Kelvin
What is the formula for density?
D= mass/volume
This number, 68200. has this many significant figures.
5
Convert 2.45 kg to g.
2450g
What are the standard units for density?
kg/m³
Convert 61.28 °C to Kelvin.
334.43 K
What is temperature?
The measure of how hot or cold an object is.
Round 4.563 to three significant figures.
4.56
Why is it important to use the SI system for measuring in science?
The world needs to use one system to easily share and interpret information/ data.
A metal block has a mass of 45.7 g, length 2.50 cm, width 1.80 cm, and height 1.20 cm. Find its density.
8.46 g/cm³
Convert 25.36 °C to Fahrenheit. Round to 2 decimal places.
77.65°F
What does daL represent?
Dekaliters
Perform the operation and round to the correct number of significant figures:
5.62 × 4.1
23
Convert 45.2 mL to L.
0.0452 L
An object has a mass of 25.6 g and fills from 7.5 mL to 32.0 mL when placed in a graduated cylinder. Find the density.
0.96 g/mL
Convert -11.98°F to Celsius.
-24.43 °C
If scientists are measuring in liters, what are they measuring?
The volume of a item
Add and round correctly: 12.45 + 3 + 0.089
16
Convert 3.571s to ns
3,571,000,000 ns
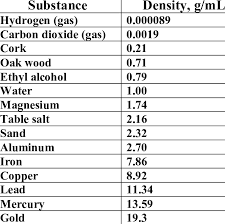
An unknown object has a mass of 10.19 g. When placed in a graduated cylinder that has a starting level of 24.0 mL, it rises to 24.75 mL of water. What is the density of this unknown object, and what substance is this object based on the chart?
Mercury- 13.59 g/mL
Convert 320.59 K to Fahrenheit.
117.39 °F
Is there a formula to convert from Kelvin to Fahrenheit? If not, how would you convert it?
First convert from Kelvin to Celsius. Then convert from Celsius to Fahrenheit.