What is the atomic number of this element?
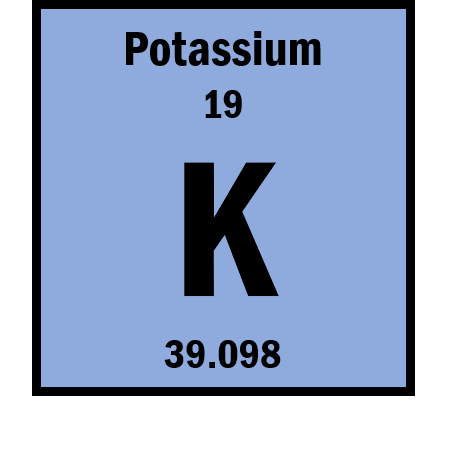
19
The most reactive side of the periodic table can be found all the way to the....
left
In a chemical equation, the arrow is read as
yields, produces
Water boiling is an example of a
physical property or physical change
An atom's valence electrons are those electrons that have the highest/lowest energy.
highest.

What is the atomic symbol?
K
The most stable side of the periodic table is found all the way to the
right
When a reaction absorbs energy instead of releasing it, it is known as an __________ reaction.
Endothermic
Substances that are produced during a chemical reaction are known as
products
Ability to react with oxygen would be a
chemical property
8
The columns in the periodic table are called
Families
When a reaction produces heat, it is known as an _______ reaction
exothermic
Substances that enter a reaction are called
reactants
The attractive force that holds two atoms together is called a(n)
bond
Lithium, sodium, potassium, are all examples of what type of element?
Alkali metals
The rows in the periodic table are called
Periods
The number written in front of chemical equations is known as the
coefficient
Give an example of a physical change
Folding, crumpling, ripping, melting, boiling, etc...
How many valance electrons does Neon have?
8
The atomic number is the total number of _________ in the nucleus of an atom
Protons
A _____________ shows the number of valence electrons in an atom in pictorial fashion
Electron dot diagram
The principle that states that matter is neither created nor destroyed during a chemical reaction is called the
Law of Conservation of Matter
Give an example as to when you know a chemical change has occurred.
Color change, burning, etc...
When the equation Al + Br2 ----> AlBr3 is balanced, the coefficient for Al is
2