one
A compound consists of ______________ kinds of atoms chemically bonded to each other.
two or more
In heterogeneous mixtures, particles are distributed or spread ________________. ( Evenly or Unevenly)
unevenly.
In homogeneous mixtures, substances are ____________ distributed. ( Evenly or Unevenly)
evenly
________ is anything that takes up space and has mass.
Matter
What is the smallest unit of an element that maintains all the properties of that element?
Atoms
Which of the these is a compound?
A. Helium
B. Hydrogen Peroxide
C. Gold
B. Baking Soda
True or false: heterogenous mixtures can be a mixture of solids, liquids, and/or gases.
True.
Mixtures can be any combination of all states of matter: solids, liquids, and gases.
True or false: Gatorade is a homogenous mixture
true
All matter is made up of ___________________.
atoms
Where are the electrons, protons, and neutrons
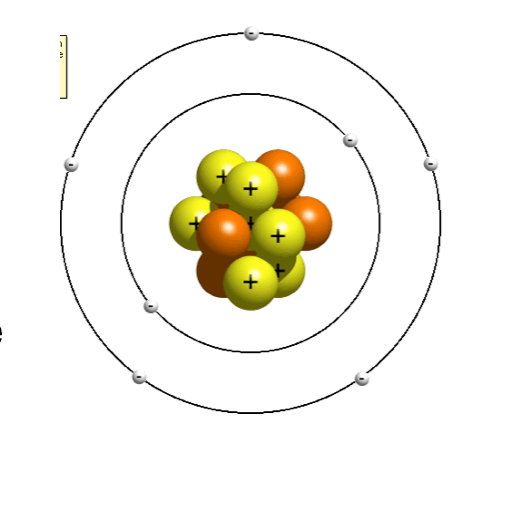
The electrons are on the outside, the protons and neutrons are in the middle
Calculate the atomic mass of NH ₃
Given that the masses of
nitrogen is 14 g/mol
hydrogen is 1 g/mol
17 g/mol
Provide 3 examples of heterogeneous mixtures.
vegetable soup, oil and water, cereal, muddy water, salad etc
Provide 3 examples of homogeneous mixtures.
koolaid, brass, salt water, lemonade etc
What are atoms?
Atoms are the building blocks of matter, sort of how bricks are the building blocks of houses.
Where you will find all elements.
Periodic table of elements
Which picture represents an element and which one represents a compound.
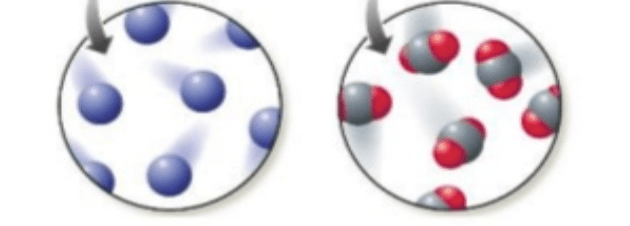
The picture fit the left are one element because it shows the atoms from one substance. The picture on the left is a compound because it shows bonded atoms.
If I have a water with snow from a snowglobe, a caesar salad, and, trail mix for lunch, which of the these are heterogeneous mixtures? (answer can be more than one).
All of the above.
Does mixing oil and water create a homogeneous mixture? Why or why not?
Mixing oil and water creates a heterogeneous mixture because we can see the oil and the water.
All __________ are made up of one type of substance, NOT 2 substances mixed together.
pure substances
Why do some elements have different letters than their symbols?
The elements are derived from Greek and Latin
Which of these statements are true about compounds? ( you may choose more than one)
A. Compounds can be found in the periodic table.
B. Compounds are pure substances.
C. Compounds are made up of two or more pure substances.
D. Compounds can be separated by physical means.
B and C
Compounds are pure substances and are made up of two or more elements.
*Elements can be found in the periodic table.
*Compounds can only be separated by chemical means.
In Brain Stew, the chemist made two mixtures. One mixture was charcoal and water. the other mixture was _______ and water.
Celite and water
What is another term we can use for homogeneous mixtures? (Hint it contains a solute and solvent.)
Solution
True or false? Both elements and compounds are pure substances
True
What does an atomic number tell you?
How many protons or neutrons an element has
What is this compound?
H₂O₂
Hydrogen Peroxide
In Brain Stew, the chemist used a _____ to separate the mixtures
filter
In homogeneous solutions, separate particles are not visible because one _______ in the other
dissolves
All _________ are ___________, but not all _________ are _____________
What does pasteurize mean?
It means to heat food over a short period of time to kill bacteria