one
A compound consists of ______________ kinds of atoms chemically bonded to each other.
two or more
In heterogeneous mixtures, particles are distributed or spread ________________. ( Evenly or Unevenly)
unevenly.
In homogeneous mixtures, substances are ____________ distributed. ( Evenly or Unevenly)
evenly
________ is anything that takes up space and has mass.
Matter
What is the smallest unit of an element that maintains all the properties of that element?
Atoms
A compound differs from a mixture in that a compound always has a
a. Homogeneous composition
B. Heterogenous composition
c. Minimum of three components
D. maximum of three components
A.
True or false: heterogenous mixtures can be a mixture of solids, liquids, and/or gases.
True.
Mixtures can be any combination of all states of matter: solids, liquids, and gases.
You observe that a sample of clear liquid has evaporated but left a powder like solid residue in the bottom of the container. how this liquid would most likely be classified.
a solution / homogenous mixture
Joshan did an experiment to differentiate between a homogenous mixture and a heterogeneous mixture. In beaker A he mixed 10g of salt to 50g of water and in beaker B he mixed 10g of flour to 50g of water. What is the product in the beakers called?
a solution
Where are the electrons, protons, and neutrons
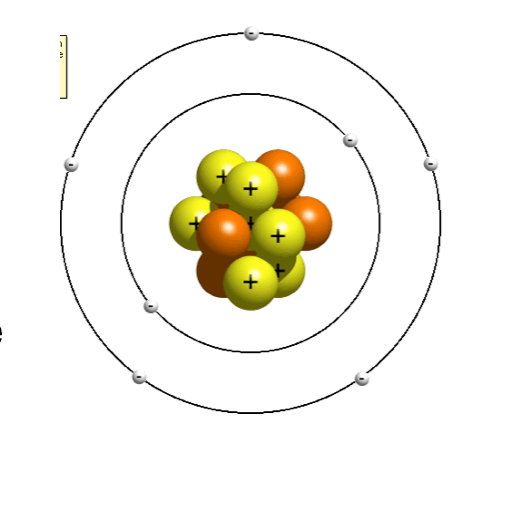
The electrons are on the outside, the protons and neutrons are in the middle
2Na + Cl2 ----> 2NaCl
The-______ sodium combines with the_______ chlorine to form the________ sodium chloride.
A. Compound; Compound; Element.
B. Element; Compound; Mixture
C. Element; Element; Compound.
D. Compound; Element; Mixture
C. Element, element , compound
Provide 2 examples of heterogeneous mixtures.
vegetable soup, oil and water, cereal, muddy water, salad etc
Provide 2 examples of homogeneous mixtures where the solute is a gas and the other the solute is a solid.
the air, koolaid in water,
David added chlorine to his swimming pool after cleaning it. What type of mixture did he create and what type of change took place
a homogenous mixture and a physical change
Atomic number of an element is determined by the __________
Number of protons
Which picture represents an element and which one represents a compound.
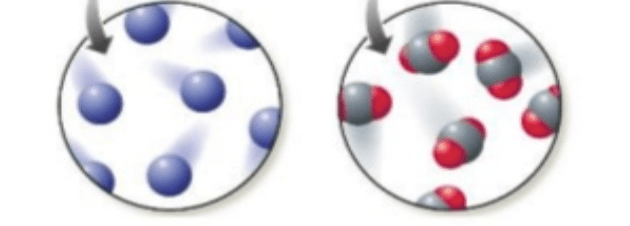
The left shows one element because it shows the atoms from one substance. The picture on the right is a compound because it shows bonded atoms.
If I have a water with snow from a snowglobe, a caesar salad, and, trail mix for lunch, which of the these are heterogeneous mixtures? (answer can be more than one).
All of the above.
Does mixing oil and water create a homogeneous mixture? Why or why not?
Mixing oil and water creates a heterogeneous mixture because we can see the oil and the water.
All __________ are made up of one type of substance, NOT 2 substances mixed together.
pure substances
Why is water NOT considered an element?
Water has 2 different atoms (elements) that are chemically combined.
Which of these statements are true about compounds? ( you may choose more than one)
A. Compounds can be found in the periodic table.
B. Compounds are pure substances.
C. Compounds are made up of two or more pure substances.
D. Compounds can be separated by physical means.
B and C
Compounds are pure substances and are made up of two or more elements.
*Elements can be found in the periodic table.
*Compounds can only be separated by chemical means.
What defines a mixture?
A. A mixture can be broken down into its basic substances through physical means.
B. A mixture cannot be broken down into its basic substances through physical means.
C. A mixture is created through a chemical reaction
D. A mixture is described by a chemical formula
A.
What is another term we can use for homogeneous mixtures? (Hint it contains a solute and solvent.)
Solution
Pure substances have fixed melting and boiling point, which of the following are pure substances.
A. Both elements and compounds are pure substances
B. only elements are pure substances
C. neither elements nor compounds are pure substances
D. elements compounds and mixtures are pure substances
A. both elements and compounds are pure substances
How many atoms are represented in the formula CO2?
3
State any two differences between a compound and a mixture
compounds can not be separated by physical means while mixtures can
Compounds are pure substances, mixtures are not
Compounds are chemically combined while mixtures are physically combined
Substances in mixtures retain their original properties while substances in compounds do not
Air is a mixture of about 78% nitrogen and 21% oxygen. Would air be a heterogeneous or homogeneous mixture? Why?
Homogenous mixture, the different gases cannot be distinguished
In homogeneous solutions, separate particles are not visible because one _______ in the other. For example, salt will __________ into water creating a saline solution.
dissolves
With mixtures all _________ are mixtures, but not all _________ are _____________
All solutions are mixtures, but not all mixtures are solutions