one
A compound consists of ______________ kinds of atoms chemically bonded to each other.
two or more
In heterogeneous mixtures, particles are distributed or spread ________________. ( Evenly or Unevenly)
unevenly.
In homogeneous mixtures, substances are ____________ distributed. ( Evenly or Unevenly)
evenly
Which of the following is the element?
a. H20
b C02
c. H
d. FeN
The smallest particle of all matter is ?
The atom.
Which of the these is a compound?
A. Helium
B. Carbon Dioxide
C. Gold
D. Silver
B. 1 Carbon + 2 Oxygen
True or false: heterogenous mixtures can be a mixture of solids, liquids, and/or gases.
True.
Mixtures can be any combination of all states of matter: solids, liquids, and gases.
In homogeneous solutions, separate particles are not visible because one _______ in the other
dissolves
Which of the following is the compound?
a. Sweet tea
b Salad
c. H
d. FeN
D
What type of letter represents an element?
Capital Letter
What is this compound - H20?
Water
Provide 2 examples of heterogeneous mixtures.
vegetable soup, oil and water, cereal, muddy water, salad etc
Provide 2 examples of homogeneous mixtures.
water, lemonade, vinegar, milk
Which of the following is the homogenous mixture?
a. Salad
b Sweet tea
c. H
d. FeN
B
How many different elements do you see in the following equation CH3PbNiCl
5
Which picture represents an element and which one represents a compound.
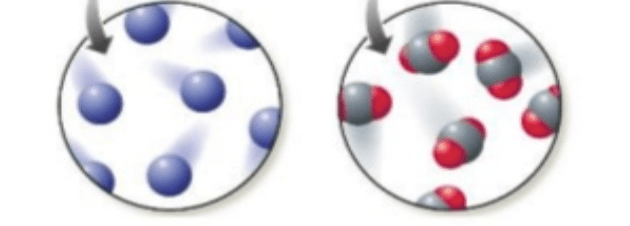
The picture fit the left are one element because it shows the atoms from one substance. The picture on the left is a compound because it shows bonded atoms.
What kind of mixture is a frito pie and why?
heterogeneous, because you can separate the fritos, chili and cheese.
Does mixing oil and water create a homogeneous mixture? Why or why not?
Mixing oil and water creates a heterogeneous mixture. Oil floats on water. It has a lower density.
Which of the following is the heterogenous mixture?
a. H20
b Pizza
c. Sweet Tea
d. Fe
B
Which of the following is NOT an element, and what is it?
1. Ca
2. Cl
3. LiFe
4. He
3, its a compound
Which of these statements are true about compounds?
A. Compounds can be found in the periodic table.
B. Compounds are made up of two or more pure substances.
C. Compounds can be separated by physical means.
B
*Elements can be found in the periodic table.
*Compounds can only be separated by chemical means.
True / False - In a heterogenous mixture I can see clearly through it.
False
Which of the following BEST models a homogeneous mixture?
A.
Place salt in a cup of water then stir until the particles disappear.
B.
Mix lettuce in a bowl with a mixture of other vegetables.
C.
Mix different samples of soil such as clay, silt, and sand in water then allow the particles to settle overnight.
D.
Separate iron filings from baking soda using a magnet.
A
What are the following examples of below and why?
a. H20
b Hamburger
c. H
d. Air
a. Compound, because its two elements combined
b. Heterogenous Mixture because you can separate the meat, dressing, bun, tomato, lettuce, pickle etc
c. Element because it is only one capital letter
d. Homogenous mixture because it looks the same throughout, its evenly mixed