What is a pure substance?
A substance comprised of only ONE thing
What are the two main types of mixtures?
Heterogeneous & Homogeneous
A type of change that does NOT result in modifying the underlying chemical structure of substances.
Physical change
The particles that make up matter.
What are atoms?
The three* states of matter.
Solid, Liquid & a Gas
Does the following image depict a pure substance?
Why or why not?
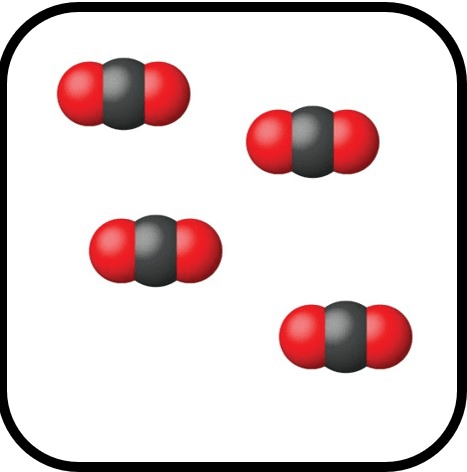
Yes.
Same compound throughout.
What is the correct description of this substance?

Heterogeneous Mixture
What type of change is iron melting in a furnace?
Support your answer.
Physical Change
Change of State
Does the following depict a compound?
Why or why not?
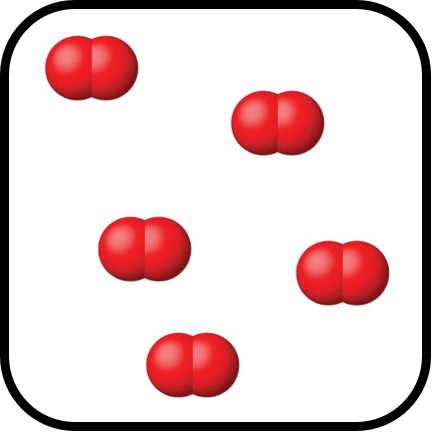
No.
Only 1 element involved in the groups.
What happens to particles when they are frozen into a solid?
They are tightly packed and vibrate, however they cannot move.
The same result.
They have uniform composition and properties.
What is the correct description of this substance?

Homogeneous Mixture
What are 3 indicators of chemical changes?
Change in color, Formation of gas, Formation of precipitate, Change in odor, Change in temperature
The three sub-atomic particles that atoms are made with.
Protons, Neutrons, Electrons
What state of matter is most likely displayed in this image?
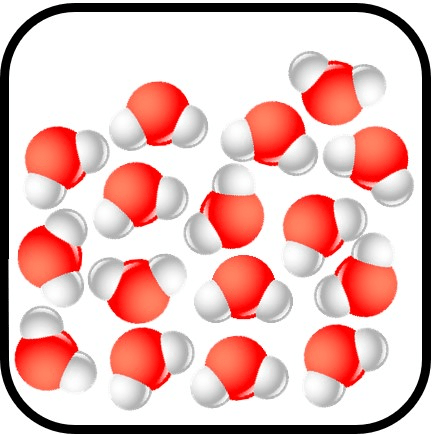
Liquid
Explain.
No, it is a mixture.
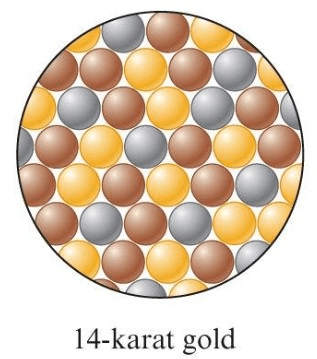
A special category of mixture that has metallic properties.
What is an alloy?
If two substances are mixed together and produce bubbles, what do we KNOW about the chemical composition of the substances during this process?
They have changed.
What is the difference between a molecule, and a compound?
Molecules are groups of 2 or more ATOMS.
Compounds are groups of 2 or more ELEMENTS.
What happens to the particles of an ice cube when they are heated up?
The particles vibrate more quickly, and then break their bonds.
A substance does NOT contain a variable composition, but it DOES contain various types of atoms.
Is this a pure substance?
Explain.
Yes.
Compounds can be pure substances.
What is a solution?
If Blue food dye is mixed with water and changes its color, what type of change does this indicate?
Physical Change
The change in color is result of physical mixing not change in chemistry.
Define how hydrogen peroxide aligns with the terms: Molecule and Compound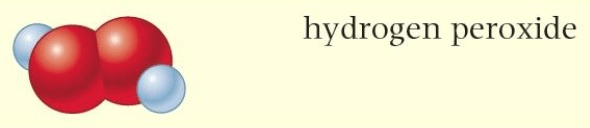
Hydrogen peroxide is BOTH a molecule and a compound.
A sample of water is heated from 50°F to 75°F.
Describe the change experienced by these molecules at the molecular level.
Speeding up, but no change in state.