Which state of matter is the most dense?

What is the chemical formula for butane, shown here?
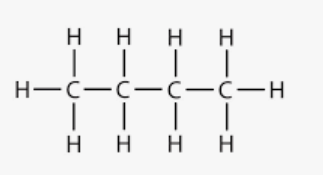
C4H10
Is water (H2O) an element, compound, or mixture?
Compound
What property allows you to identify copper as reddish-brown compared to aluminum’s silver-gray appearance?
Color
What is the term for a substance that contains more than one atom?
A molecule
What is the formula for density?
density = mass/volume
How many hydrogen atoms are in C6H12O6?
12
Is oxygen gas (O2) an element, compound, or mixture?
Element
What property identifies water because it always boils at 100°C at standard atmospheric pressure?
Boiling point
What is the term for a substance that has different types of atoms bonded together?
A compound
A cube has a mass of 60 g and a volume of 20 mL. What is its density?
3 g/mL
Write the chemical formula of adamantane with 10 carbon atoms and 16 hydrogen atoms.
C10H16
Is this a mixture of elements or a mixture of compounds, or both?
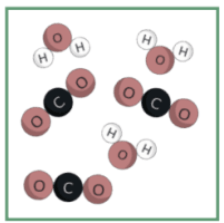
Mixture of compounds
What property allows you to identify substances like gasoline or wood because they burn easily?
What is the term a substance that contains different molecules next to each other, but not bonded together?
A mixture
An iron nail has a mass of 40 g and a volume of 5 mL. What is its density?
8 g/mL
Write the chemical formula for the molecule shown below:
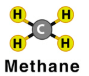
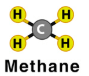
2CH4
A diamond is made of all carbon atoms. Is it an element, compound, or mixture?
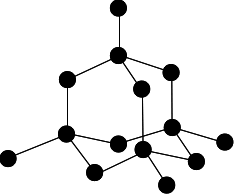
Element
What property helps you identify vinegar because it has a strong, sour smell?
Odor
What is the term for a substance that is all made of the same atom?
An element
Water's density is 1 g/mL. Glycerin has a density of 1.26 g/mL. Will glycerin float?
No
Write the chemical formula for the molecule shown below:
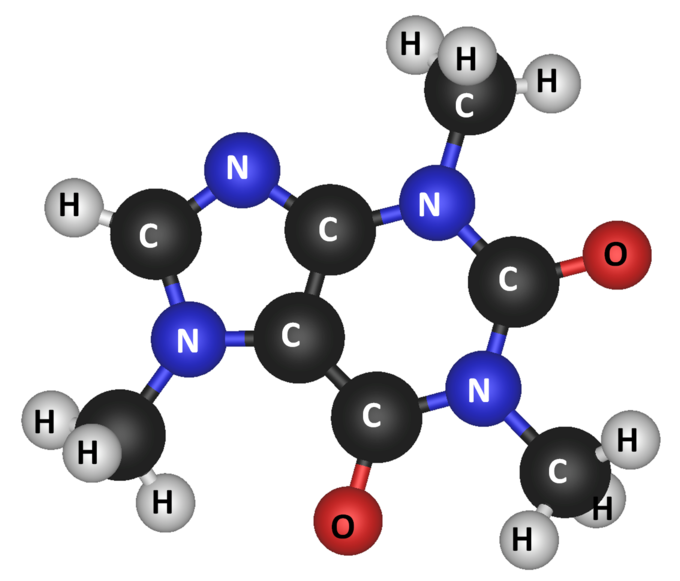
C8H10O2N4
Is this a mixture of elements or a mixture of compounds, or both?
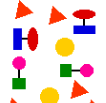
Both
What property helps you identify table salt because it dissolves easily in water, while sand does not?
Solubility
What is the term for the amount of matter in an object?
Mass