This word means:
Able to be dissolved (especially in water).
Soluble
True or False? Plastic is insoluble in water.
TRUE
Is the following reaction balanced or unbalanced?
Al + O2 --> Al2O3
Unbalanced
This word means:
Ratio between the mass and the amount of substance (measured in moles) of any sample of said compound
Molar Mass
When a given material is able to react with something, this is a chemical or physical change?
Chemical
What is the molar mass of C6H2O6?
170.08 g/mol
Units for molar mass
Grams per mol or g/mol
What type of reaction is this?
3 H2 (g) + N2 (g) --> 2 NH3 (g)
Synthesis
Convert 2.50 moles of NaCl to grams.
146 grams
This word means:
A standard scientific unit for measuring large quantities of very small entities such as atoms, molecules, or other specified particles.
Mole or Mol
These are examples of what type of reaction?
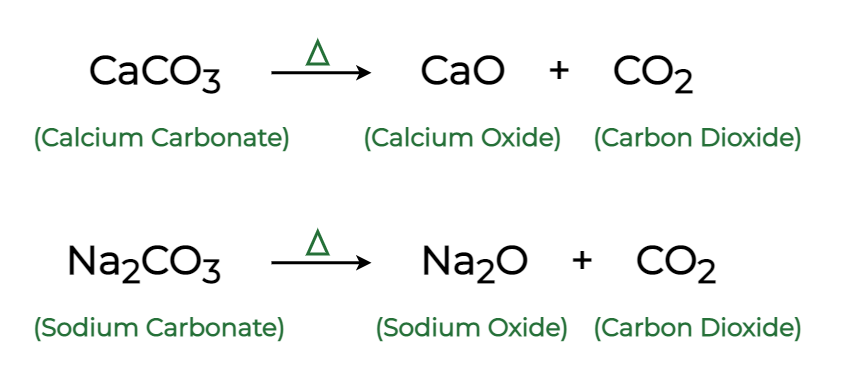
Decomposition
Balance the following chemical reaction:
C2H6 + O2 --> CO2 + 2 H2O
2 C2H6 + 7 O2 + 4 CO2 + 6 H2O
Avogadro's Number
(correctly)
6.022 x 1023
This is the number of units in a mole.
True or False? 1 mol of NaCl weighs the same as 1 mol of I2.
TRUE
Use the equation for the following reaction:
2 KClO3 --> 2 KCl + 3 O2
If 1.50 moles of KClO3 decomposes, how many grams of O2 will be produced?
72.0 g O2